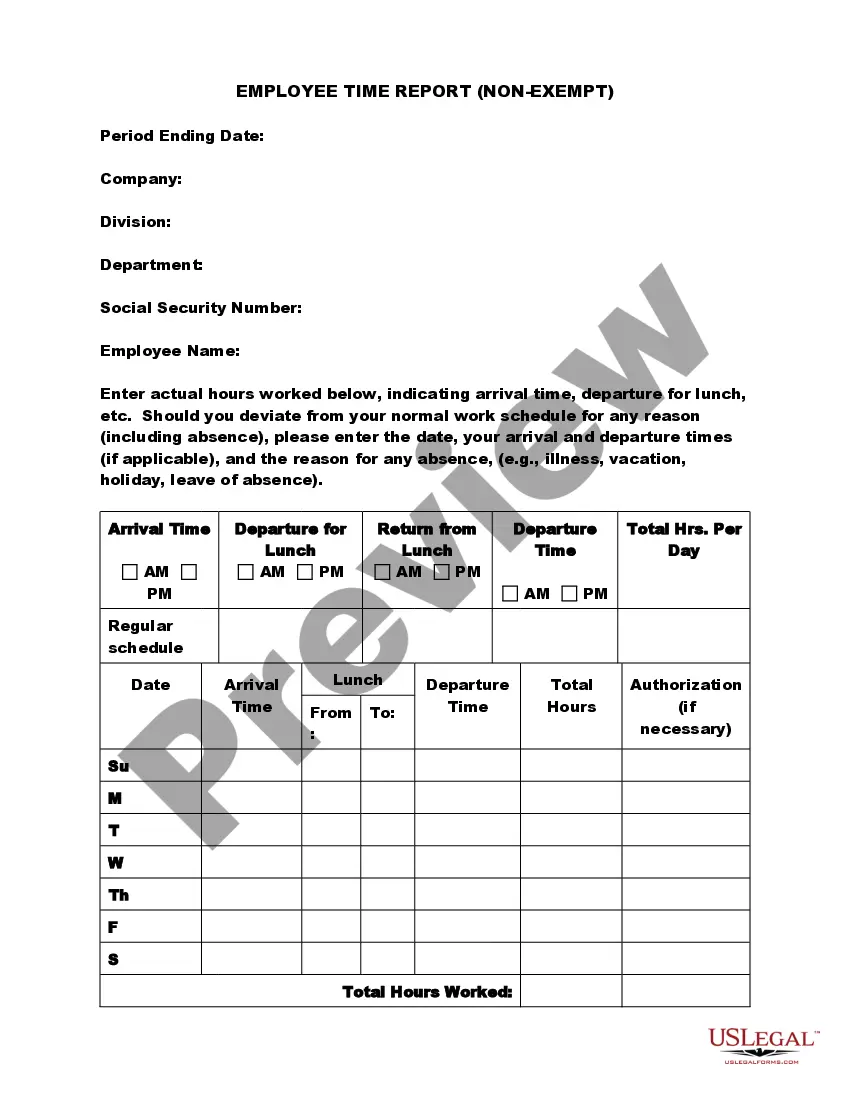
New Hampshire Monitored Time Info and Pamphlet - Off Clock Pitfalls
Description
How to fill out Monitored Time Info And Pamphlet - Off Clock Pitfalls?
You can spend multiple hours online attempting to discover the legal document template that satisfies the state and federal requirements you need.
US Legal Forms provides a vast array of legal forms that are vetted by experts.
It is easy to download or print the New Hampshire Monitored Time Info and Pamphlet - Off Clock Pitfalls from the service.
If available, utilize the Preview button to review the document template as well.
- If you already possess a US Legal Forms account, you can Log In and click on the Obtain button.
- Then, you can complete, modify, print, or sign the New Hampshire Monitored Time Info and Pamphlet - Off Clock Pitfalls.
- Each legal document template you obtain is yours indefinitely.
- To retrieve another copy of the bought form, navigate to the My documents section and click the relevant button.
- If you are using the US Legal Forms website for the first time, follow the simple instructions below.
- First, ensure that you have selected the correct document template for the state/city of your choice. Read the form summary to confirm that you have chosen the appropriate form.
Form popularity
FAQ
15 calendar days is the timeline given for reporting a SUSAR (LT/Fatal).
For serious and unexpected, but non-fatal adverse events, file as soon as possible and no later than 15 days after initial receipt of the SAE. All SAEs must be reported to the IRB within 5 business days as "reportable new information."
Investigators are required to report promptly to the IRB2026 all unanticipated problems involving risks to human subjects or others, including adverse events that should be considered unanticipated problems (21 CFR 56.108b1, 21 CFR 312.53c1vii, and 21 CFR 312.66).
A SUSAR that meets the seriousness criteria of life-threatening and/or results in death must be reported within seven (7) calendar days. A SUSAR that is not life-threatening or does not result in death must be submitted to the regulatory authorities within fifteen (15) calendar days.
An SAE that occurs during research with a medicinal product may be a SAR or a SUSAR. SAR is the abbreviation for Serious Adverse Reaction, and SUSAR for Suspected Unexpected Serious Adverse Reaction.
The minimum dataset required to consider information as a reportable AE is indeed minimal, namely (1) an identifiable patient, (2) an identifiable reporter, (3) product exposure, and (4) an event.
The criteria for a valid case are: an identifiable patient; 25cf a suspect drug; 25cf a suspect reaction; 25cf an identifiable HCP reporter.
The California Department of Public Health (CDPH) and Medi-Cal both mandate the reporting of events within 5 Days of the event's discovery.
Investigator reporting: notifying the study sponsor This section also describes the use of a Serious Adverse Event Form as the document for recording and reporting such events.
If you think you or someone in your family has experienced a serious reaction to a medical product, you are encouraged to take the reporting form to your doctor. Your health care provider can provide clinical information based on your medical record that can help FDA evaluate your report.