North Dakota Electron Medical Device Certification Request is a process for obtaining certification for medical devices in the state of North Dakota. The certification request must include documentation and information about the device that will be certified and is used to ensure that the device meets safety and performance requirements. There are two types of North Dakota Electron Medical Device Certification Requests: Class I and Class II. Class I requests are for non-invasive and non-life-supporting devices, whereas Class II requests are for invasive and life-supporting devices. To request certification, the manufacturer must provide documentation such as device specifications, safety tests, and detailed instructions for use. After the request is approved, the device must be registered with the North Dakota Department of Health.
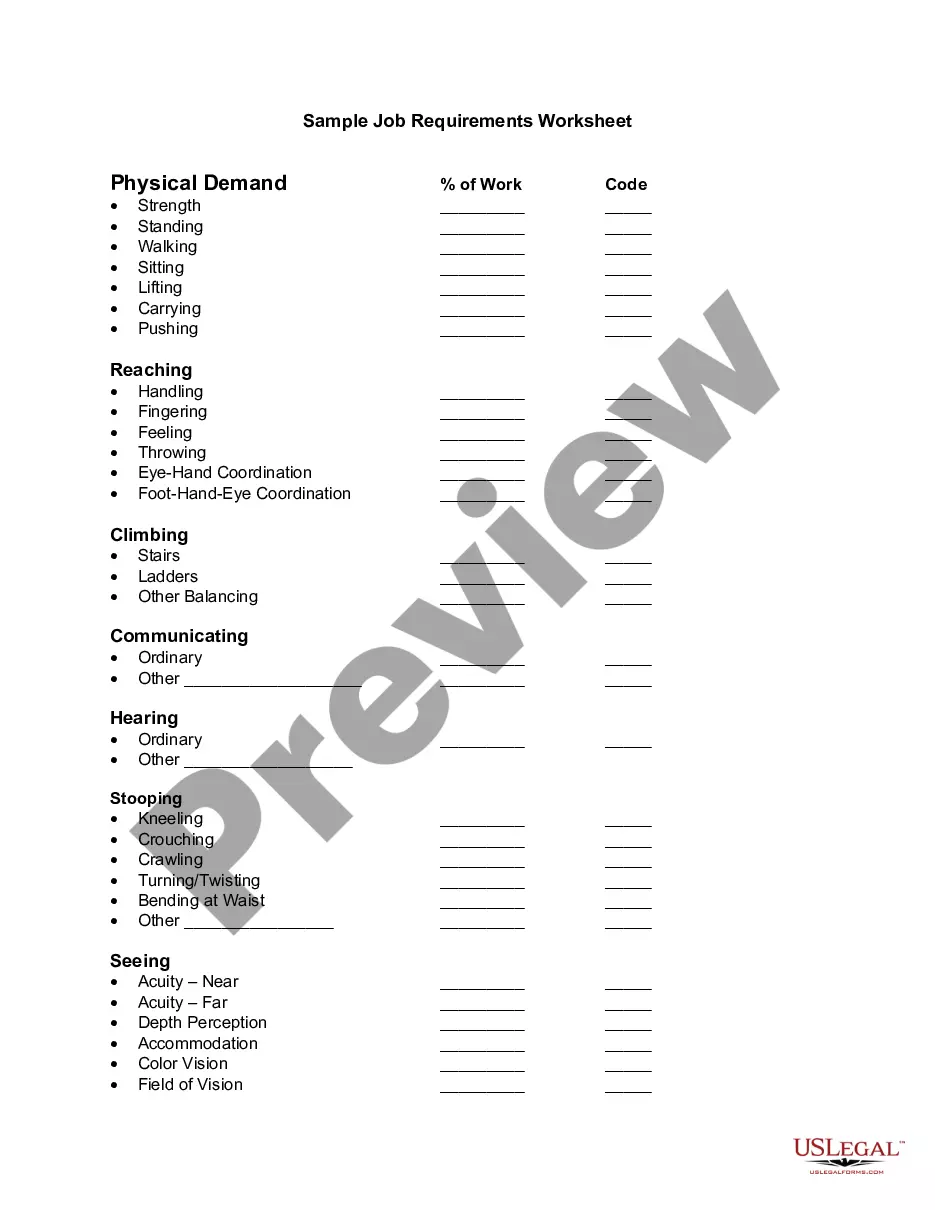
North Dakota Electro Medical Device Certification Request
Description
Get your form ready online
Our built-in tools help you complete, sign, share, and store your documents in one place.
Make edits, fill in missing information, and update formatting in US Legal Forms—just like you would in MS Word.
Download a copy, print it, send it by email, or mail it via USPS—whatever works best for your next step.
Sign and collect signatures with our SignNow integration. Send to multiple recipients, set reminders, and more. Go Premium to unlock E-Sign.
If this form requires notarization, complete it online through a secure video call—no need to meet a notary in person or wait for an appointment.
We protect your documents and personal data by following strict security and privacy standards.
Make edits, fill in missing information, and update formatting in US Legal Forms—just like you would in MS Word.

Download a copy, print it, send it by email, or mail it via USPS—whatever works best for your next step.

Sign and collect signatures with our SignNow integration. Send to multiple recipients, set reminders, and more. Go Premium to unlock E-Sign.
If this form requires notarization, complete it online through a secure video call—no need to meet a notary in person or wait for an appointment.
We protect your documents and personal data by following strict security and privacy standards.
Looking for another form?
How to fill out North Dakota Electro Medical Device Certification Request?
How much time and resources do you usually spend on composing official paperwork? There’s a better opportunity to get such forms than hiring legal specialists or wasting hours browsing the web for an appropriate blank. US Legal Forms is the leading online library that offers professionally designed and verified state-specific legal documents for any purpose, such as the North Dakota Electro Medical Device Certification Request.
To get and complete an appropriate North Dakota Electro Medical Device Certification Request blank, follow these simple instructions:
- Look through the form content to ensure it complies with your state laws. To do so, check the form description or use the Preview option.
- In case your legal template doesn’t satisfy your needs, locate another one using the search bar at the top of the page.
- If you already have an account with us, log in and download the North Dakota Electro Medical Device Certification Request. Otherwise, proceed to the next steps.
- Click Buy now once you find the right document. Select the subscription plan that suits you best to access our library’s full opportunities.
- Sign up for an account and pay for your subscription. You can make a transaction with your credit card or via PayPal - our service is totally safe for that.
- Download your North Dakota Electro Medical Device Certification Request on your device and complete it on a printed-out hard copy or electronically.
Another advantage of our service is that you can access previously acquired documents that you securely keep in your profile in the My Forms tab. Pick them up at any moment and re-complete your paperwork as often as you need.
Save time and effort completing legal paperwork with US Legal Forms, one of the most trusted web services. Join us now!
Form popularity
FAQ
North Dakota uses Medicaid managed care for most expansion enrollees, but 19- and 20-year-olds are covered under fee-for-service Medicaid.
Eligibility Provide services to at least one North Dakota Medicaid eligible recipient. Meet the conditions regulating the specific type of provider, program, and/or service. Hold a current license, certification, accreditation, or registration ing to North Dakota state laws and regulations.
Medicaid Expansion Coverage Toll-Free: (833) 777-5779 / 711 (TTY) - This number is now accepting calls from Medicaid Expansion members.
Client Share (Recipient Liability) is the amount of monthly net income remaining after all appropriate deductions, disregards, and Medicaid income levels have been allowed. All such income must be considered to be available for payment of medical services provided to the eligible individual or family.
If you need assistance, please contact Provider Enrollment at (701) 277-6999 during business office hours from Monday to Friday 8 am - pm CST.
Payer List Payer NamePayer IDTypeND MedicaidNDMCDmedicaidND Medicare Part B03302medicareNE BCBS00760bcbsNE MedicaidSKNE0medicaid94 more rows