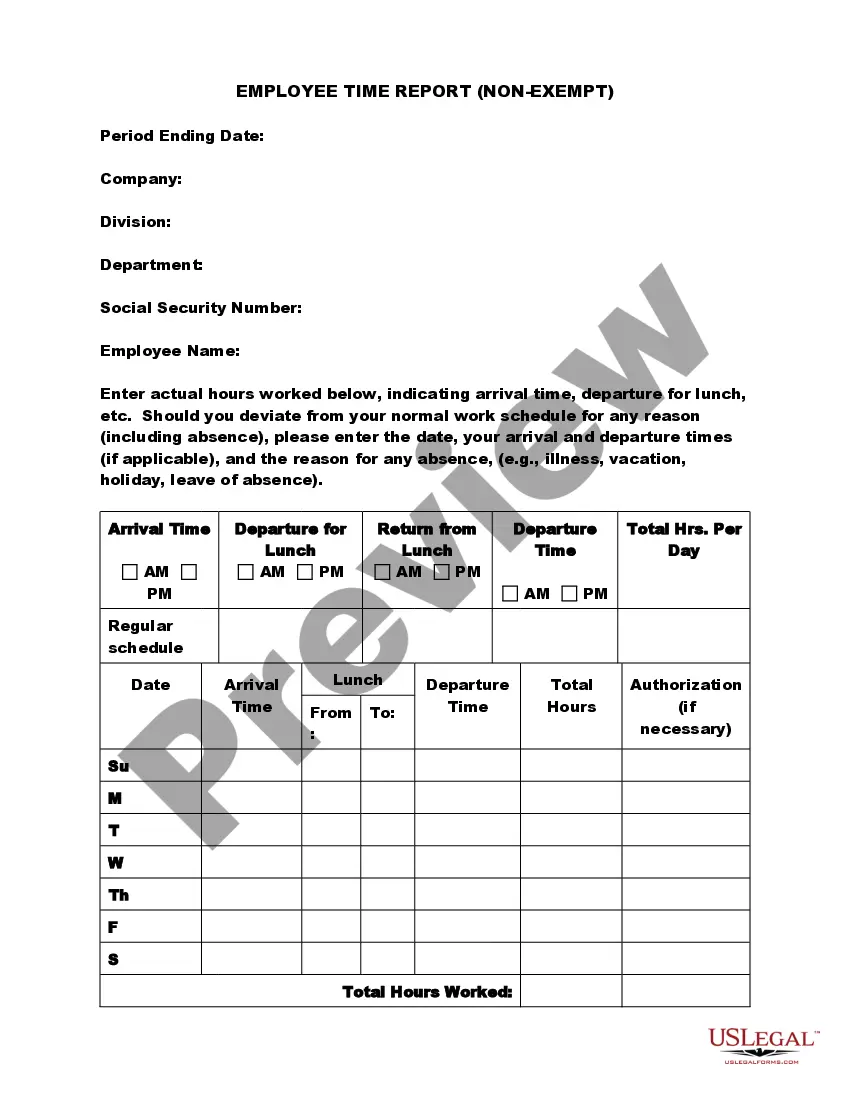
Iowa Exempt Survey
Description
How to fill out Exempt Survey?
US Legal Forms - one of the largest collections of legal documents in the USA - offers a variety of legal document templates that you can download or print.
By using the website, you can access thousands of forms for business and personal use, organized by categories, states, or keywords. You will find the latest versions of forms like the Iowa Exempt Survey in just minutes.
If you have a monthly subscription, Log In to download the Iowa Exempt Survey from the US Legal Forms library. The Download button will appear on each form you view. You can find all previously saved forms in the My documents tab of your account.
Process the payment. Use a credit card or PayPal account to complete the transaction.
Choose the format and download the form to your device. Make modifications. Fill out, edit, print, and sign the downloaded Iowa Exempt Survey. Every template you added to your account has no expiration date and is yours permanently. Therefore, if you wish to download or print another copy, just go to the My documents section and click on the form you need. Access the Iowa Exempt Survey with US Legal Forms, one of the most comprehensive libraries of legal document templates. Utilize thousands of professional and state-specific templates that cater to your business or personal needs.
- Ensure you have selected the correct form for your city/region.
- Click on the Review button to check the form's content.
- Read the form description to ensure you have chosen the right form.
- If the form does not meet your needs, use the Search field at the top of the page to find one that does.
- If you are satisfied with the form, confirm your choice by clicking the Purchase now button.
- Then, select the pricing plan you prefer and provide your details to sign up for the account.
Form popularity
FAQ
Research regulated by the Food and Drug Administration (FDA): With the exception of Category 6, FDA-regulated research does not qualify for exempt status.
If a study is granted exemption from IRB review, the Board does not require consent documentation but the Board generally requires that participants receive information about the study.
Emergency research is the most often mentioned example in all contexts described, and it is the most notable research discipline in which practical problems are forwarded as the primary reason why the informed consent requirement should be waived.
The HHS regulations at 45 CFR part 46 for the protection of human subjects in research require that an investigator obtain the legally effective informed consent of the subject or the subject's legally authorized representative, unless (1) the research is exempt under 45 CFR 46.101(b); (2) the IRB finds and documents
Federal regulations outline formal informed consent requirements for non-exempt research. Research confirmed as meeting the criteria for exempt review (i.e. exempt research) is not subject to those formal requirements, allowing PIs flexibility in how informed consent is obtained.
All research conducted on humans must be pre-emptively accepted by the subjects themselves through the procedure known as informed consent. Voluntary expression of consent and adequate information disclosure about the research are critical and essential elements of the informed consent process.
Yes, in some circumstances. The HHS regulations require that an investigator obtain legally effective informed consent from subjects or a legally authorized representative before the subjects may be involved in research (45 CFR 46.116), unless this requirement has been waived by an IRB.
An informed consent document is typically used to provide subjects with the information they need to make a decision to volunteer for a research study. Federal regulations (45 CFR 46.116 ) provide the framework for the type of information (i.e., the "elements") that must be included as part of the consent process.
As indicated above, use of a formal, signed consent form is not required by the IRB for studies under exempt review. For many benign surveys, a few simple paragraphs preceding the survey sufficiently meets the spirit of this ethical tenet.