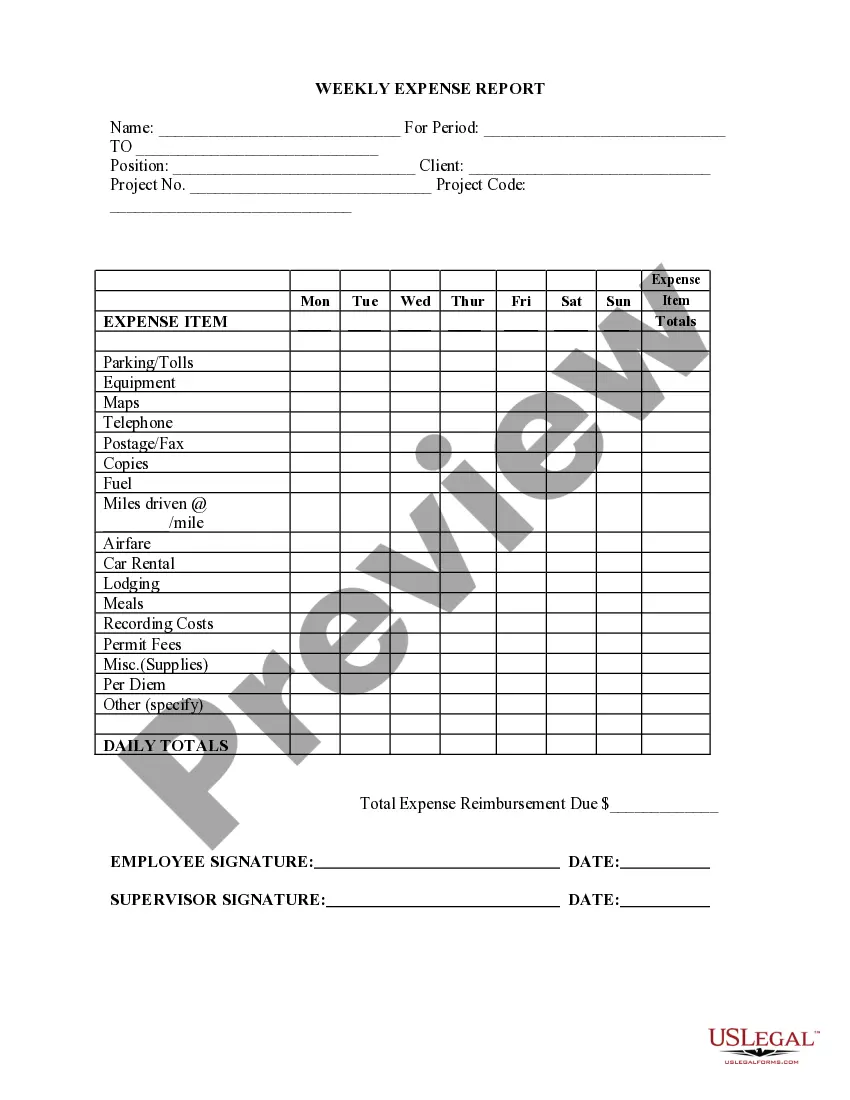
Consent Form For Assignment In Washington
Description
Form popularity
FAQ
5 things everyone should know about consent Consent comes first. Before engaging in any sexual activity, it's necessary to establish consent. Consent is fluid. Consent can be verbal or physical. Incapacitation doesn't count. Work together to improve your experience.
The consent form should describe if/when identifiable data will be destroyed and how such data will be protected and how it will be used or shared. Language - Consent forms should be written in the 2nd person (i.e., "you are") and in a language that is clear, concise, and understandable to the subject population.
With reference to the subject mentioned above, I Father/ Mother / Guardian of (Name of the student), Class/Sec. Roll No. Student ID. hereby pleased to give my consent and allow my ward to attend the school / institute for classes and related activities.
Using this strategy, legal, regulatory, philosophical, medical, and psychological literatures have come together to support the following elements of informed consent: (1) disclosure, (2) understanding, (3) voluntariness, (4) competence, and (5) consent (see National Commission 1978; Meisel and Roth 1981; President's ...
Informed Consent Checklist (1998) A statement that the study involves research. An explanation of the purposes of the research. The expected duration of the subject's participation. A description of the procedures to be followed. Identification of any procedures which are experimental.
A statement that the study involves research, an explanation of the purposes of the research and the expected duration of the subject's participation, a description of the procedures to be followed, and identification of any procedures which are experimental.
I consent to participate in the research project and the following has been explained to me: the research may not be of direct benefit to me. my participation is completely voluntary. my right to withdraw from the study at any time without any implications to me.
Basic Elements of Informed Consent Purpose of the Research. Description of the Research. Risks. Benefits. Alternatives to Participation.
Considerations in preparing the informed consent document: Elements of consent present. Complete explanations. Lay language. Protection of confidentiality. No unproven claims of effectiveness. Device studies include a statement that the study includes an evaluation of the safety of the test article.
State the Purpose: Mention the letter's purpose and what you consent to. Be specific about the details. Provide Details: Include any relevant details about the consent, such as dates, locations, and conditions. Sign and Date: End with your signature and date.