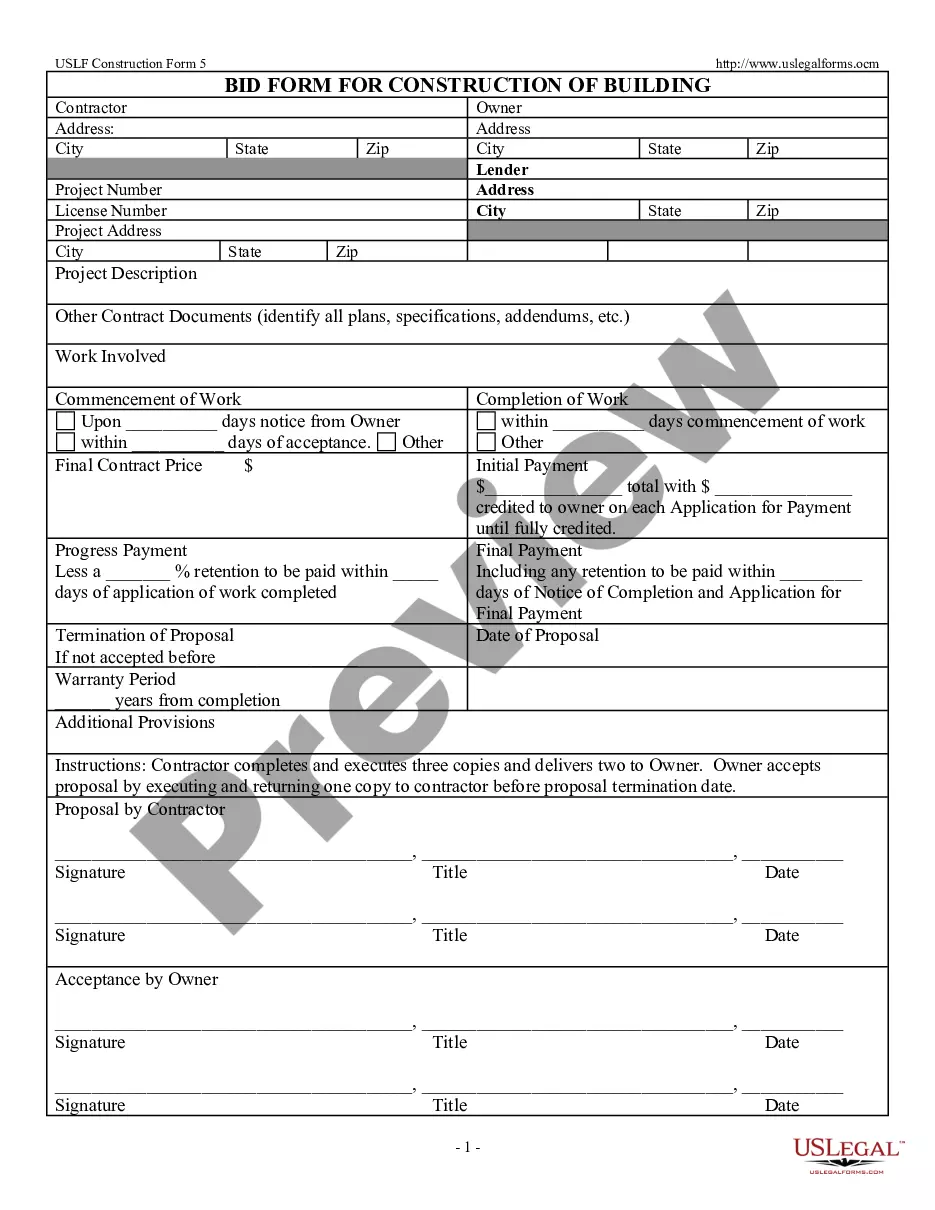
Consent Form For Assignment In Salt Lake
Description
Form popularity
FAQ
How to write a consent form: A step-by-step guide Step 1: Title and introduction. Step 2: Description of the activity. Step 3: Risks and benefits. Step 4: Confidentiality and data handling. Step 5: Voluntary participation and withdrawal. Step 6: Consent statement. Step 7: Signature and date. Step 8: Contact information.
Documenting informed consent occurs after explaining the research and assessing participant comprehension. At minimum, it involves obtaining the signature of the participant (or the legally-authorized representative or parent(s), when approved) as well as the person obtaining consent.
Informed consent is documented by the use of a written consent form approved by the IRB and signed and dated by the subject or the subject's legally authorized representative at the time of consent. A copy of the signed and dated consent form must be given to the person signing the form.
I agree to take part in this study as a research participant. By my signature I affirm that I am at least 18 years old and that I have received a copy of this Consent and Authorization form.
With reference to the subject mentioned above, I Father/ Mother / Guardian of (Name of the student), Class/Sec. Roll No. Student ID. hereby pleased to give my consent and allow my ward to attend the school / institute for classes and related activities.
If you prefer to write your own consent document, you may do so, but be sure to include all required elements of informed consent.
General instructions Write in a conversational tone. Who is your audience? ... Directly address the participant. Avoid legalistic language (e.g. you hereby agree, you certify that, etc.) Use bullet point lists to increase readability. Use a readable font such as Arial, Courier, or Verdana.
Documenting informed consent occurs after explaining the research and assessing participant comprehension. At minimum, it involves obtaining the signature of the participant (or the legally-authorized representative or parent(s), when approved) as well as the person obtaining consent.
To create a consent form, follow these steps: State the purpose of the consent form and why consent is needed. Describe the activity or procedure in detail and outline potential risks. Explain that participation is voluntary and that information will be kept confidential.
If you plan to use an electronic consent process, you will need to explain the process of documenting consent in your protocol and provide a copy of the electronic consent form. An electronic consent form needs to cover all the required consent information as provided in the Electronic Consent Template.