Consent Form For Assignment In Suffolk
Description
Form popularity
FAQ
To make it a click-n-sign consent form, follow these steps: From within Simple Practice at the calendar screen, click My Account (look for the gear icon) > Settings > Notes and Forms (on the left). Click the blue button "Create New Template > Create New Template."
Obtaining Written or Verbal Informed Consent. Obtaining consent involves explaining the research and assessing participant comprehension using a consent document, usually a written consent form or information sheet, as a guide for the verbal explanation of the study.
The subject or the subject's legally authorized representative or the parent(s) must sign the short form, and the person actually obtaining the consent must sign the copy of the summary (45 CFR 46.117(b)(2)).
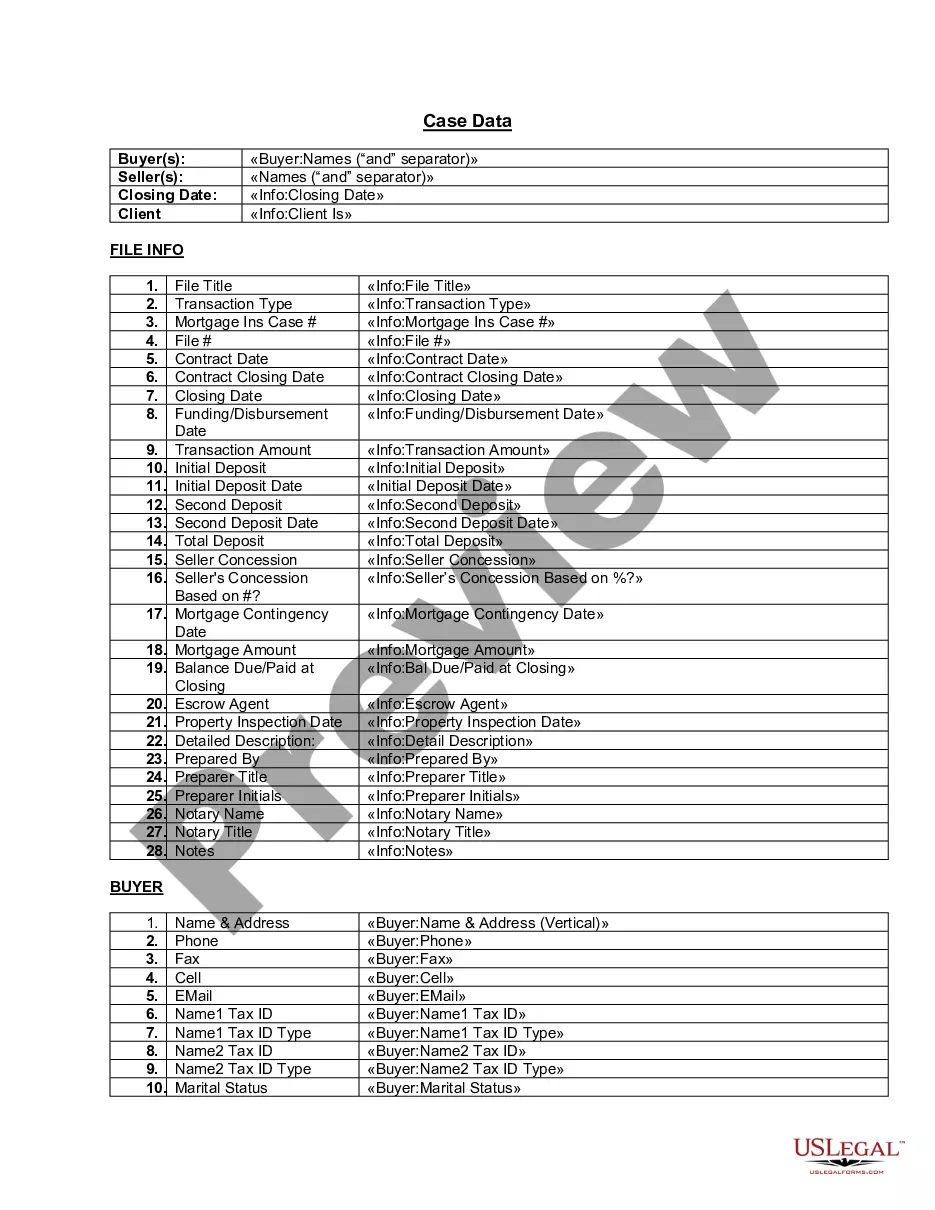
To create a consent form, you need to list the consenting parties and specify the activities or data covered by the consent. It should also state the parties' rights and responsibilities and include dates, contact information, and other necessary details.
If you prefer to write your own consent document, you may do so, but be sure to include all required elements of informed consent.
If you plan to use an electronic consent process, you will need to explain the process of documenting consent in your protocol and provide a copy of the electronic consent form. An electronic consent form needs to cover all the required consent information as provided in the Electronic Consent Template.
The consent form should include the following statements: I confirm that I have had the project explained to me, and have read the participant information sheet, which I may keep for my records. I have been given the opportunity to ask questions and have had them answered to my satisfaction.
Considerations in preparing the informed consent document: Elements of consent present. Complete explanations. Lay language. Protection of confidentiality. No unproven claims of effectiveness. Device studies include a statement that the study includes an evaluation of the safety of the test article.
State the Purpose: Mention the letter's purpose and what you consent to. Be specific about the details. Provide Details: Include any relevant details about the consent, such as dates, locations, and conditions. Sign and Date: End with your signature and date.
The consent form should include the following statements: I confirm that I have had the project explained to me, and have read the participant information sheet, which I may keep for my records. I have been given the opportunity to ask questions and have had them answered to my satisfaction.