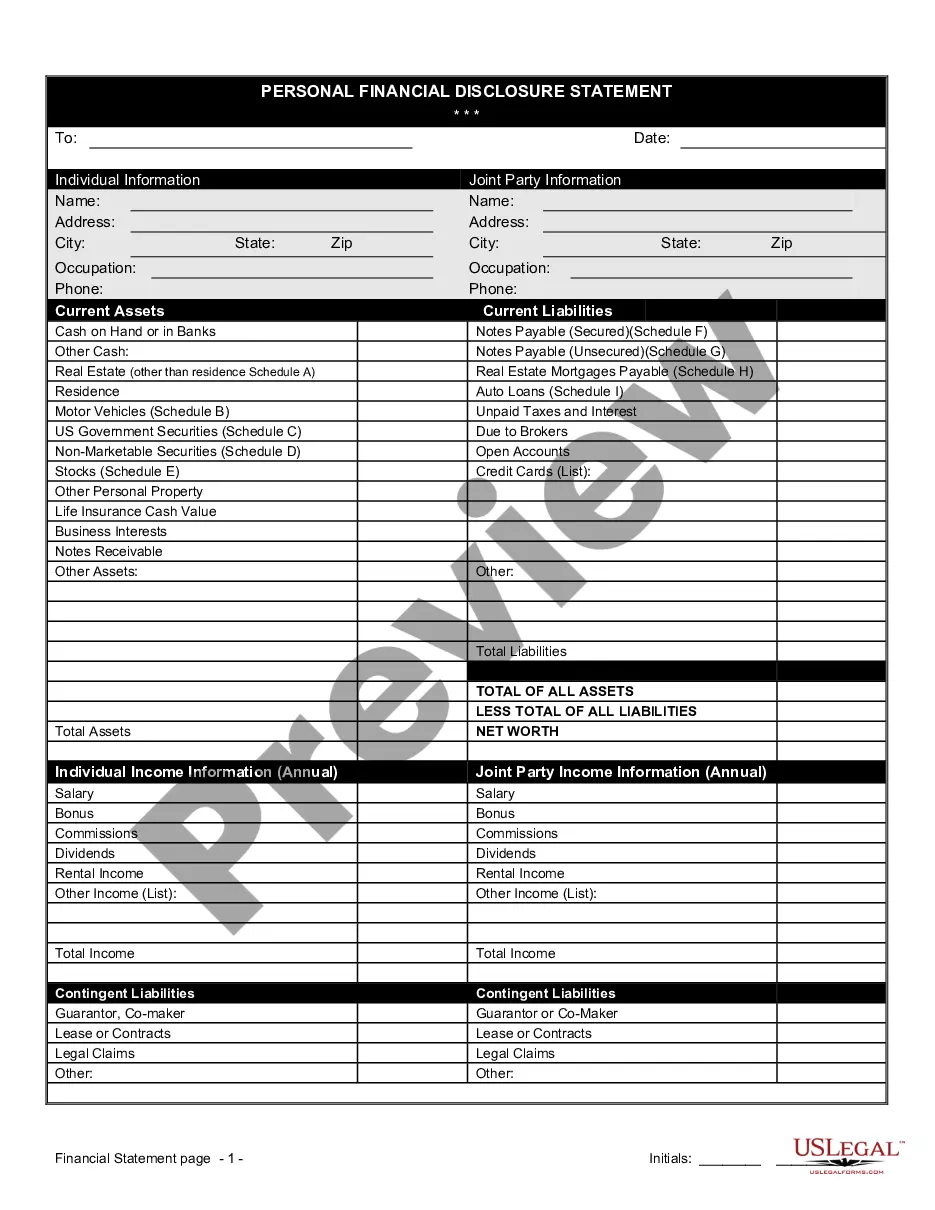
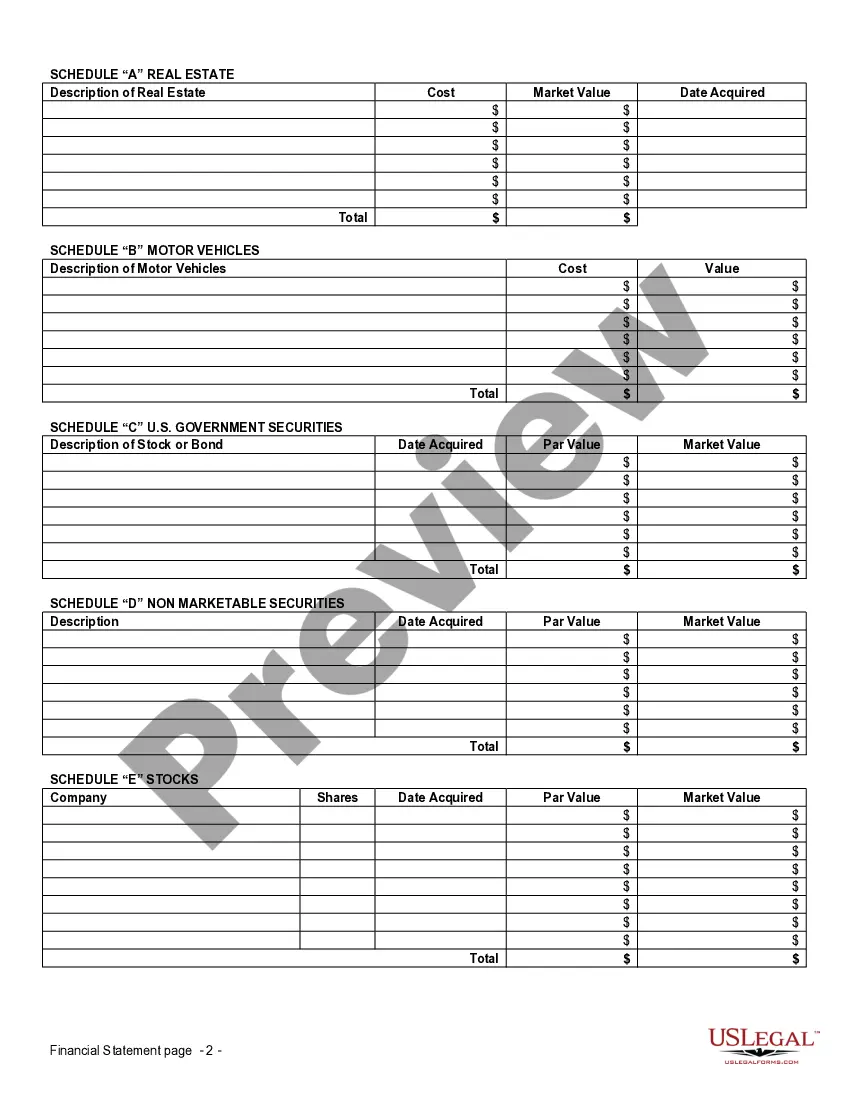
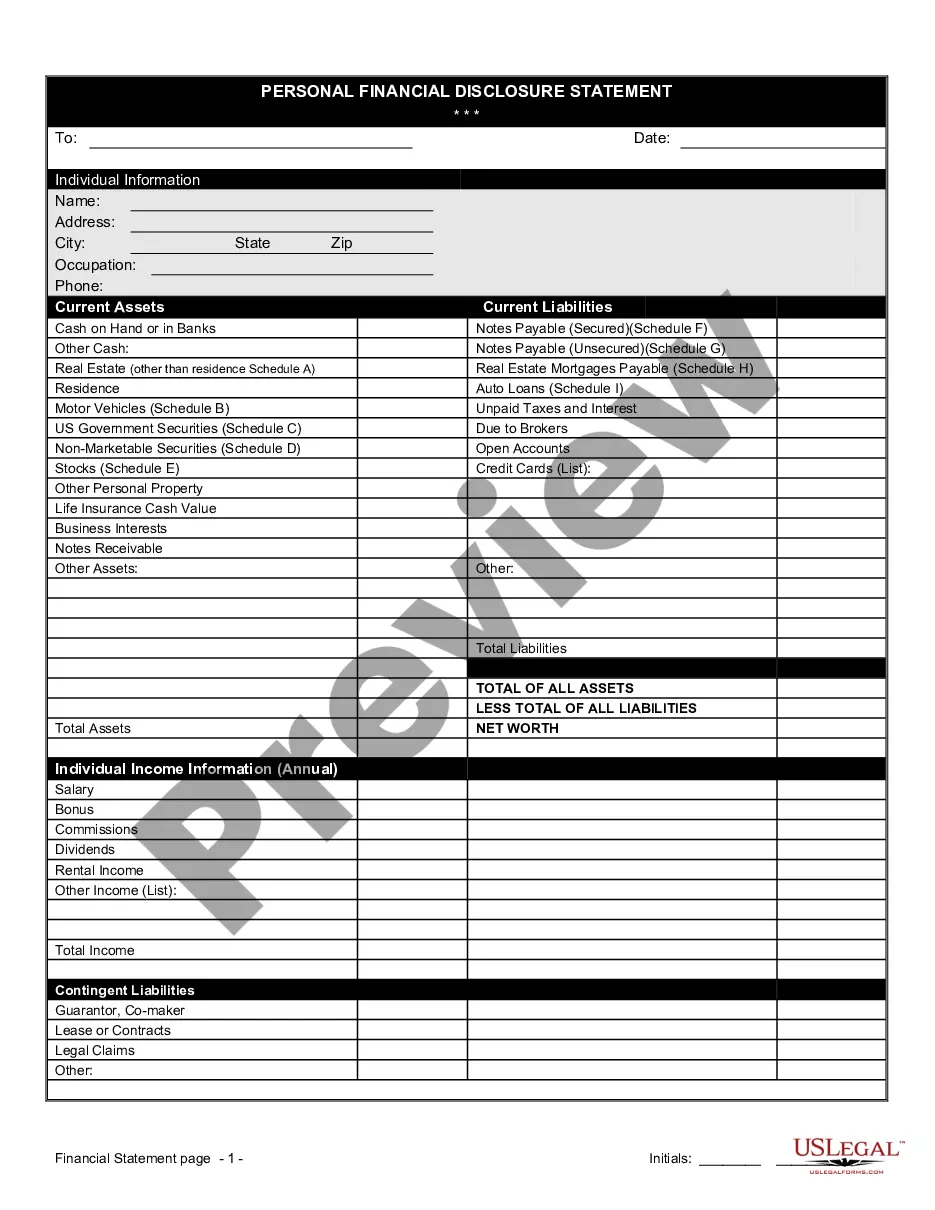
Financial Statement Form Blank For Clinical Trials
Description
How to fill out Financial Statement Form - Husband And Wife Joint?
Finding a go-to place to take the most current and appropriate legal templates is half the struggle of working with bureaucracy. Choosing the right legal documents demands accuracy and attention to detail, which explains why it is important to take samples of Financial Statement Form Blank For Clinical Trials only from reliable sources, like US Legal Forms. An improper template will waste your time and hold off the situation you are in. With US Legal Forms, you have very little to worry about. You can access and see all the information about the document’s use and relevance for your circumstances and in your state or county.
Consider the listed steps to complete your Financial Statement Form Blank For Clinical Trials:
- Use the catalog navigation or search field to locate your template.
- View the form’s description to see if it suits the requirements of your state and area.
- View the form preview, if available, to make sure the template is definitely the one you are interested in.
- Resume the search and locate the proper document if the Financial Statement Form Blank For Clinical Trials does not fit your needs.
- When you are positive regarding the form’s relevance, download it.
- If you are an authorized user, click Log in to authenticate and access your selected forms in My Forms.
- If you do not have a profile yet, click Buy now to obtain the form.
- Choose the pricing plan that fits your requirements.
- Go on to the registration to complete your purchase.
- Complete your purchase by picking a transaction method (credit card or PayPal).
- Choose the file format for downloading Financial Statement Form Blank For Clinical Trials.
- When you have the form on your gadget, you may modify it with the editor or print it and complete it manually.
Remove the headache that accompanies your legal documentation. Explore the extensive US Legal Forms catalog where you can find legal templates, check their relevance to your circumstances, and download them on the spot.
Form popularity
FAQ
Form FDA 3454, or the Financial Certification or Disclosure Statement, is used to submit information regarding clinical investigators who participated in the clinical studies. If no clinical studies were performed, simply state: ?no clinical studies were performed to test this device.?
When new investigators are assigned to a clinical investigation under an investigational new drug application (IND), the sponsor completes and signs a Form 1572 before allowing the investigator to get involved in the clinical investigation.
The FDA Form 1571 or '1571' is the IND application cover page and it must accompany the initial IND submission and any amendments, IND safety reports, annual reports or general correspondence the sponsor submits to the FDA about the IND. The 1571 is a contractual agreement between the sponsor and the FDA.
TTU OP 70.37 asks all employees and other individuals planning to act as investigators, including their spouses and dependent relatives or household members, to disclose: 1) any significant business or financial interest (SFI) that would reasonably appear to be related to the investigator's institutional ... What is an Annual Financial Interest Disclosure? | Research Home ttu.edu ? faculty ? stories ? June ? fina... ttu.edu ? faculty ? stories ? June ? fina...
§ 54.4 Certification and disclosure requirements. The applicant is required to submit for each clinical investigator who participates in a covered study, either a certification that none of the financial arrangements described in § 54.2 exist, or disclose the nature of those arrangements to the agency.