Consent Form For Assignment In San Jose
Description
Form popularity
FAQ
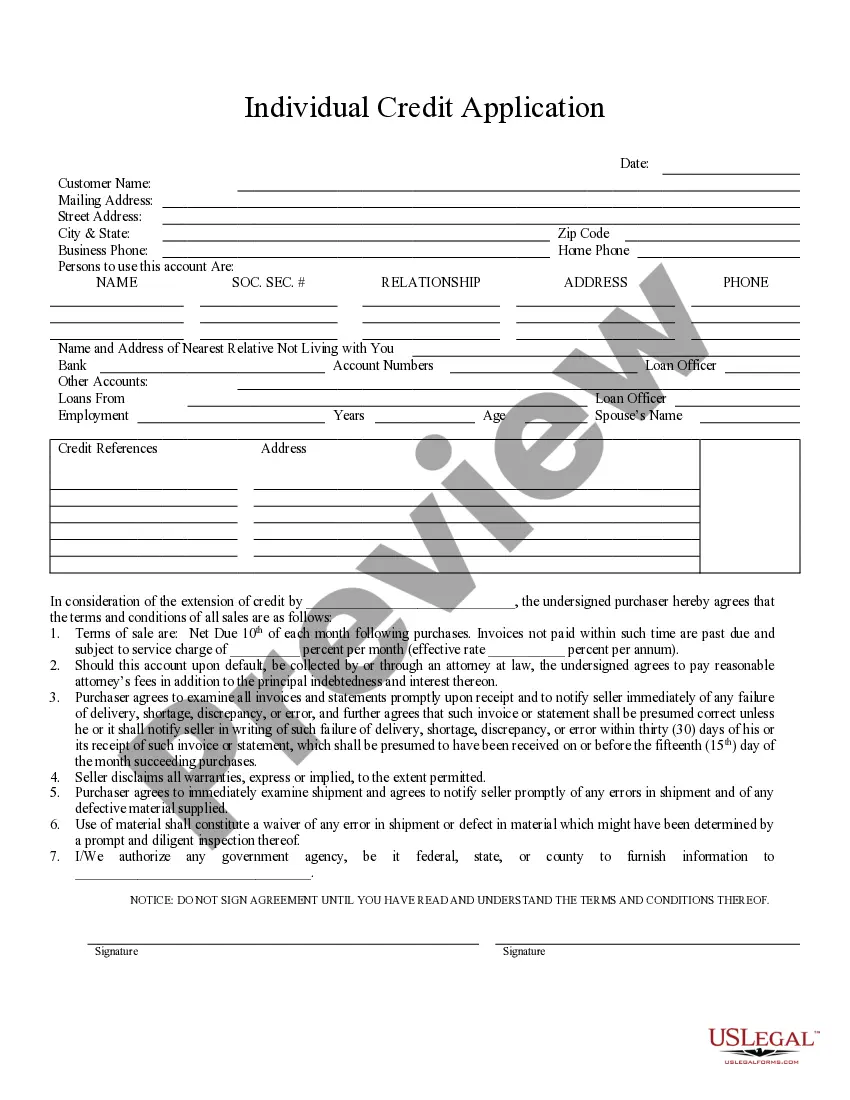
Considerations in preparing the informed consent document: Elements of consent present. Complete explanations. Lay language. Protection of confidentiality. No unproven claims of effectiveness. Device studies include a statement that the study includes an evaluation of the safety of the test article.
How to write a consent form: A step-by-step guide Step 1: Title and introduction. Step 2: Description of the activity. Step 3: Risks and benefits. Step 4: Confidentiality and data handling. Step 5: Voluntary participation and withdrawal. Step 6: Consent statement. Step 7: Signature and date. Step 8: Contact information.
A statement that the study involves research, an explanation of the purposes of the research and the expected duration of the subject's participation, a description of the procedures to be followed, and identification of any procedures which are experimental.
State the Purpose: Mention the letter's purpose and what you consent to. Be specific about the details. Provide Details: Include any relevant details about the consent, such as dates, locations, and conditions. Sign and Date: End with your signature and date.
Instructions for Developing an Informed Consent Document General Information. Describe the purpose(s) of this research study in lay terms. Purpose of the Study. Procedures. Risks. Benefits. Compensation, Costs and Reimbursement. Withdrawal or Termination from Study. Confidentiality.
If you prefer to write your own consent document, you may do so, but be sure to include all required elements of informed consent.
I participant name, agree to participate or agree to participation of my child participant name in the research project titled project title, conducted by researcher(s) name who has (have) discussed the research project with me. I have received, read and kept a copy of the information letter/plain language statement.
The consent form should describe if/when identifiable data will be destroyed and how such data will be protected and how it will be used or shared. Language - Consent forms should be written in the 2nd person (i.e., "you are") and in a language that is clear, concise, and understandable to the subject population.
I participant name, agree to participate or agree to participation of my child participant name in the research project titled project title, conducted by researcher(s) name who has (have) discussed the research project with me. I have received, read and kept a copy of the information letter/plain language statement.
I understand that my participation is voluntary and that I may withdraw my participation at any time without penalty. I voluntarily agree to participate in this study. __ I do __ I do not give you permission to make audio/video recordings of me during this study (if applicable).