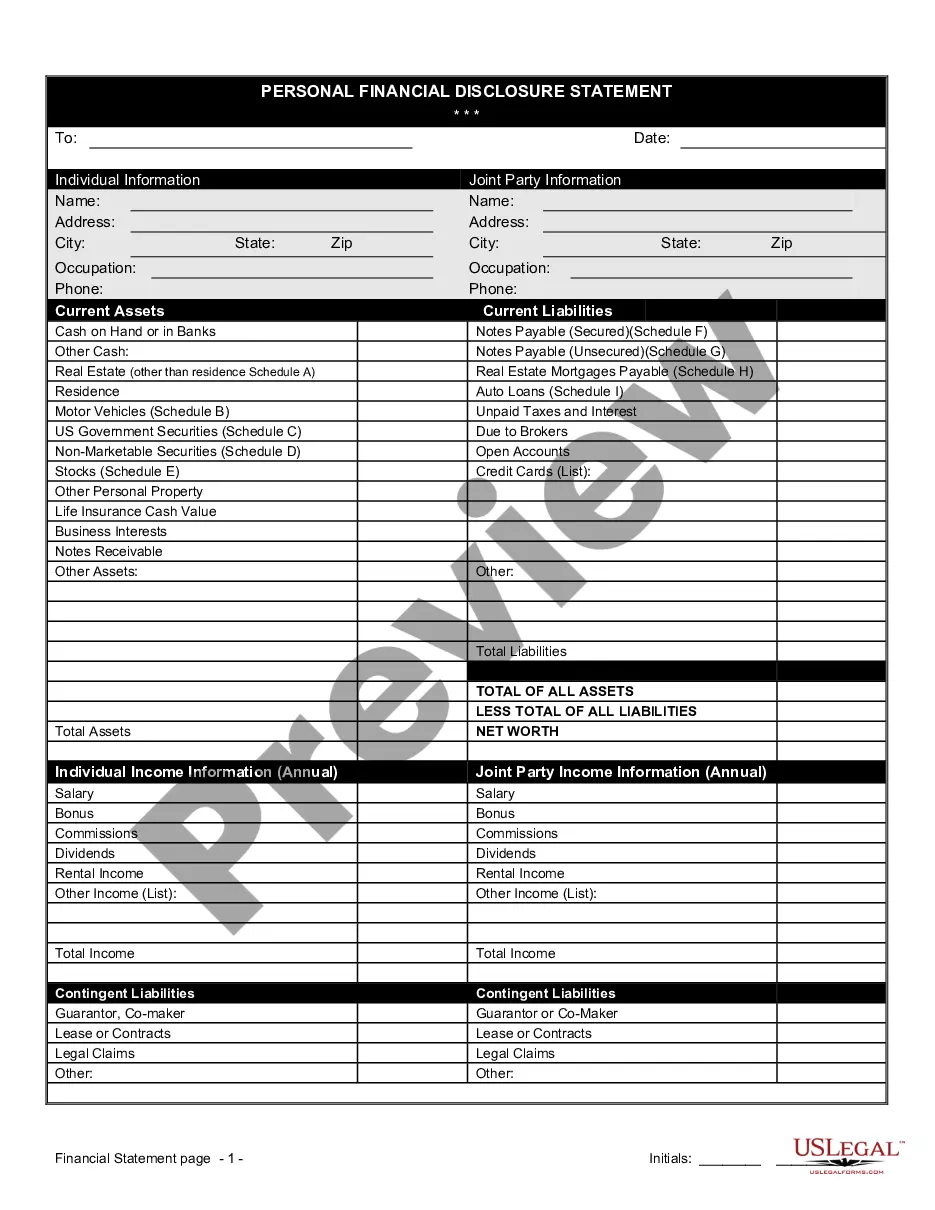
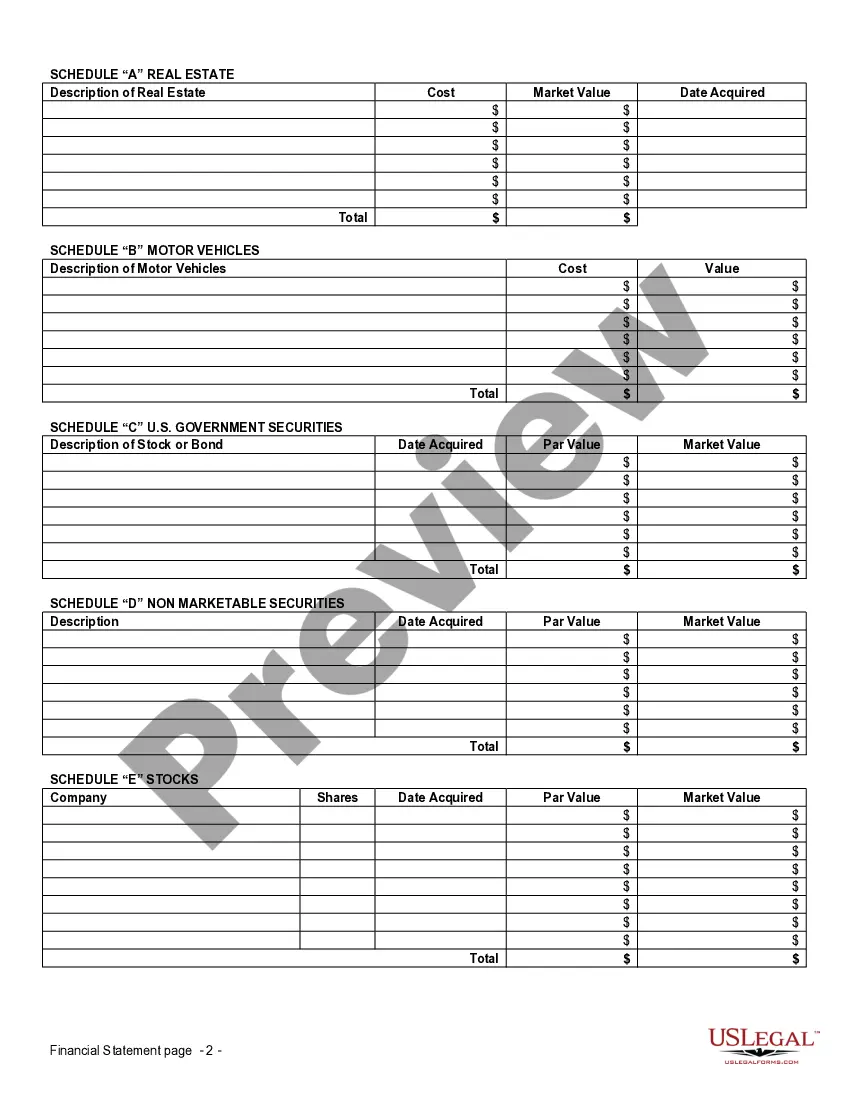
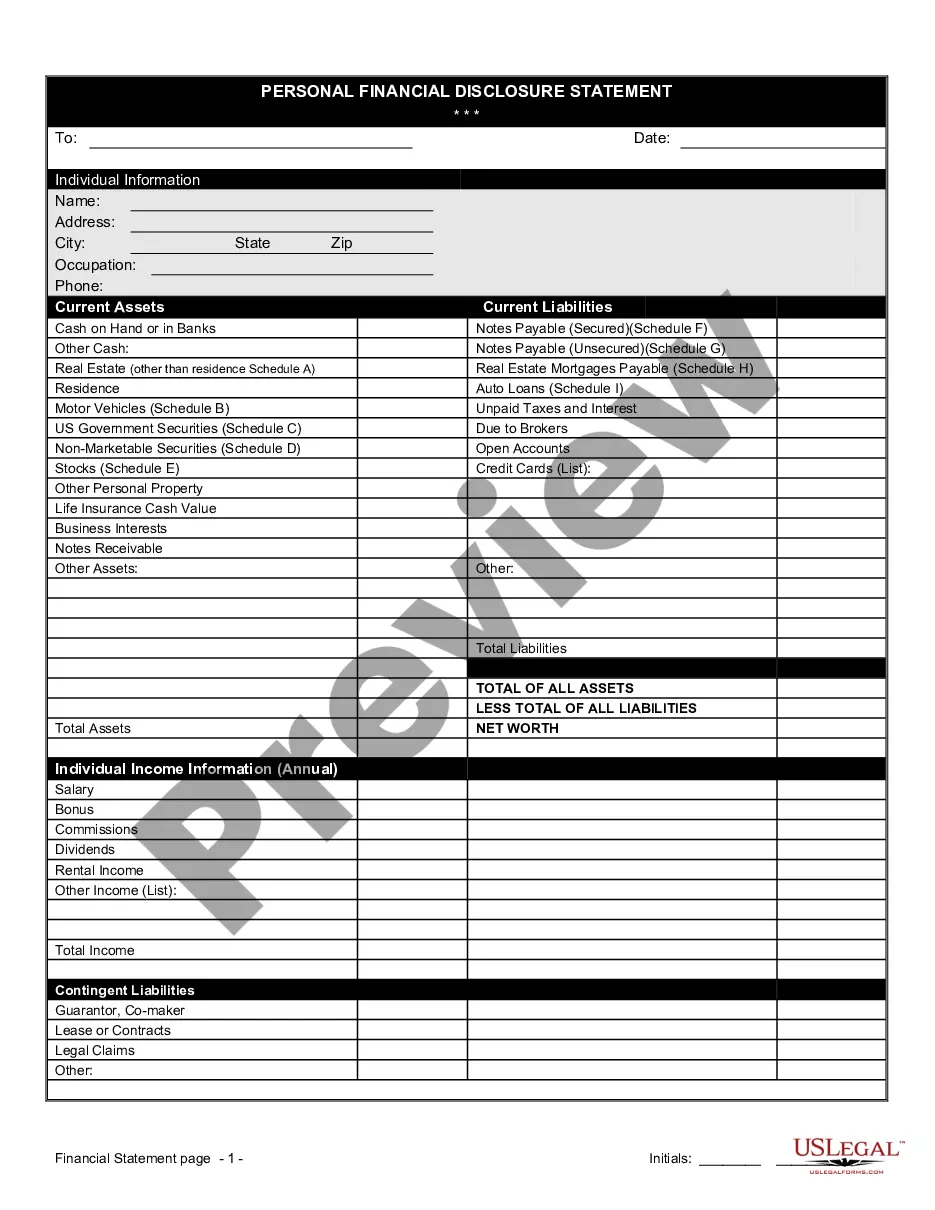
Financial Statement Form Printable For Clinical Trials
Description
How to fill out Financial Statement Form - Husband And Wife Joint?
The Financial Statement Form Printable For Clinical Trials displayed on this page is a versatile formal template crafted by qualified attorneys in accordance with federal and state statutes and guidelines.
For over 25 years, US Legal Forms has supplied individuals, enterprises, and legal professionals with more than 85,000 authenticated, state-specific documents for any business and personal event. It’s the quickest, simplest, and most reliable method to acquire the documentation you require, as the service ensures the utmost level of data protection and anti-malware safeguards.
Subscribe to US Legal Forms to have validated legal templates for all of life’s circumstances readily available.
- Explore the document you require and review it.
- Choose the pricing plan that fits your needs and create an account.
- Select the format you desire for your Financial Statement Form Printable For Clinical Trials (PDF, DOCX, RTF) and store the sample on your device.
- Complete and sign the documentation.
- Access the same document again whenever needed.
Form popularity
FAQ
FDA generally expects that applicants will be able to provide this information. Under 21 CFR §§ 312.53(c), 812.20(b)(5) and 812.43(c), a sponsor is required to obtain clinical investigator financial information before allowing the clinical investigator to participate in a covered clinical study.
Complete form 3454 if none of the investigators have any FDA required disclosures. Complete form 3455 if any clinical investigator has a financial disclosure that is significant.
TTU OP 70.37 asks all employees and other individuals planning to act as investigators, including their spouses and dependent relatives or household members, to disclose: 1) any significant business or financial interest (SFI) that would reasonably appear to be related to the investigator's institutional ... What is an Annual Financial Interest Disclosure? | Research Home ttu.edu ? faculty ? stories ? June ? fina... ttu.edu ? faculty ? stories ? June ? fina...
§ 54.4 Certification and disclosure requirements. The applicant is required to submit for each clinical investigator who participates in a covered study, either a certification that none of the financial arrangements described in § 54.2 exist, or disclose the nature of those arrangements to the agency.
Financial disclosure is the submission of information concerning compensation to, and financial interests and arrangements of, any clinical investigator conducting clinical studies.