Wayne Michigan Exempt Survey
Description
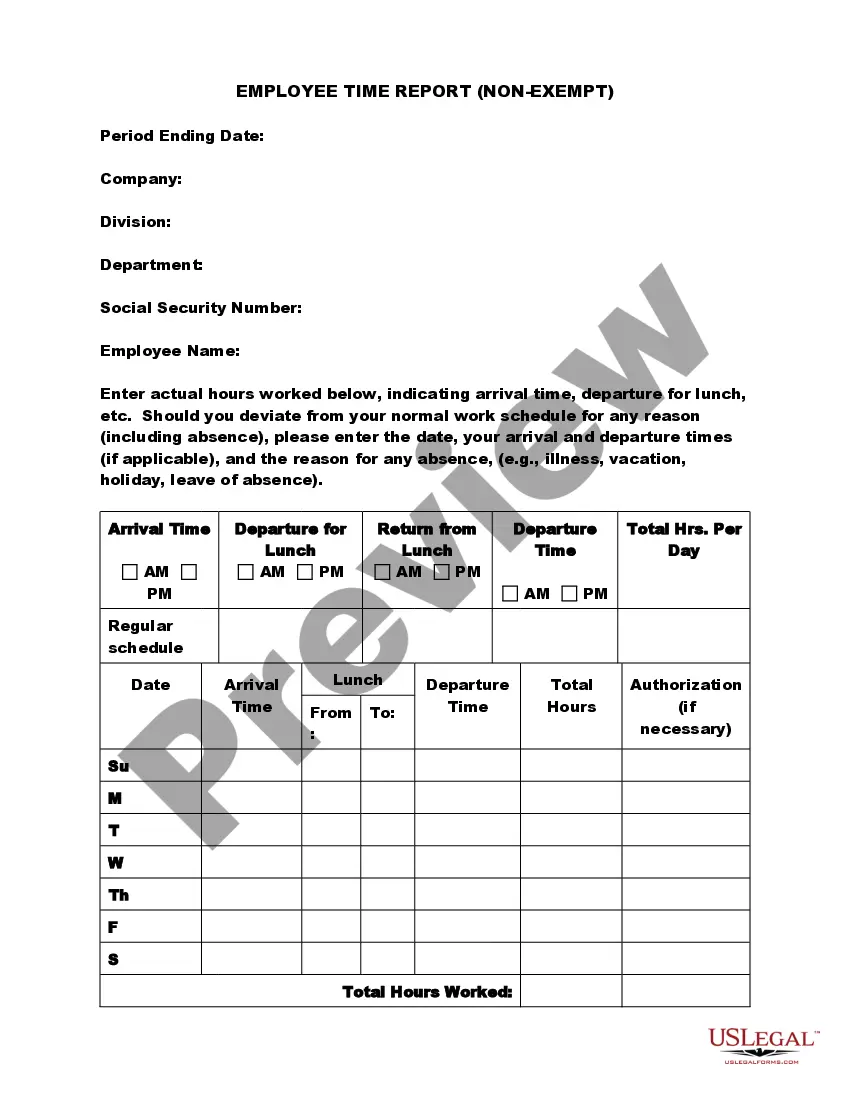
How to fill out Exempt Survey?
If you are looking to find a dependable legal document provider to acquire the Wayne Exempt Survey, think about US Legal Forms. Whether you wish to initiate your LLC enterprise or manage the distribution of your assets, we have you covered. You don’t need to be an expert in law to search for and download the necessary template.
Just select to search or browse Wayne Exempt Survey, either through a keyword or by the applicable state/county.
After finding the required template, you can Log In and download it or save it in the My documents section.
Don’t have an account? It’s simple to begin! Just find the Wayne Exempt Survey template and check the form’s preview and brief introductory details (if available). If you’re okay with the template’s language, proceed and click Buy now. Create an account and select a subscription plan. The template will be immediately ready for download once the payment is finalized.
Managing your legal issues doesn’t need to be costly or laborious. US Legal Forms is here to prove it. Our extensive array of legal forms makes these tasks more economical and accessible. Establish your first business, arrange your advance care planning, draft a real estate agreement, or finalize the Wayne Exempt Survey - all from the comfort of your sofa.
- You can explore from over 85,000 forms organized by state/county and circumstance.
- The user-friendly interface, plethora of supporting resources, and dedicated assistance simplify the process of obtaining and executing different documents.
- US Legal Forms is a reputable service supplying legal forms to millions of clients since 1997.
Form popularity
FAQ
Research activities in which the only involvement of human subjects will be in one or more of the exempt categories defined by the federal regulations, will be given an exempt determination, rather than IRB approval. Exempt studies are so named because they are exempt from some of the federal regulations.
Can parental or guardian permission for research involving children be waived? Yes, under certain circumstances. An Institutional Review Board (IRB) may waive the requirements for obtaining parental or guardian permission if it makes and documents the findings under either 45 CFR 46.116(c) or (d).
Research that involves greater than minimal risk: Research eligible for exemption usually involves negligible risks to subjects. When reviewing an application for exempt status, OPHS staff apply the ?minimal risk? standard.
A determination of exemption must be made by the JHM IRBs and principal investigators must submit all such studies for their review. Although the research may qualify as exempt, it must still be conducted in accordance with the ethical principles for human subjects research outlined in the Belmont Report. 1.
Exempt Categories: Education research. Surveys, interviews, educational tests, public observations (that do not involve children) Benign behavioral interventions. Analysis of previously-collected, identifiable info/specimens. Federal research/demonstration projects. Taste and food evaluation studies.
Of the protocols considered ?research,? the most convenient from the standpoint of the submitting principal investigator is the Exempt protocol designation. This category is defined by the Office of Human Research Protection (OHRP) to encompass research that poses no more than minimal harm to research participants.
Human subjects research that is classified as ?exempt? means that the research qualifies as no risk or minimal risk to subjects and is exempt from most of the requirements of the Federal Policy for the Protection of Human Subjects, but is still considered research requiring an IRB review for an exemption determination.
It is the policy of the Mayo Clinic Office for Human Research Protection, Institutional Review Board (IRB), that all research activities under its jurisdiction involving human subjects be reviewed to determine whether the research meets one or more exemption categories, as defined by Federal regulations.
Research involving children can be classified as exempt under this category if the research involves only educational tests and / or observation of public behavior where the investigator does not participate in the activities being observed and meets the other conditions of the exempt category.
Research can qualify for an exemption if it is no more than minimal risk and all of the research procedures fit within one or more of the exemption categories in the federal IRB regulations. Studies that qualify for exemption must be submitted to the IRB for review before starting the research.