Nebraska Authorization to Use or Disclose Protected Health Information
Description
How to fill out Authorization To Use Or Disclose Protected Health Information?
You might spend hours online trying to discover the legal template that satisfies the state and federal stipulations you require.
US Legal Forms offers a vast array of legal documents which are validated by experts.
You can easily download or print the Nebraska Authorization to Use or Disclose Protected Health Information from the platform.
If available, utilize the Review button to examine the template as well.
- If you already have a US Legal Forms account, you can Log In and press the Download button.
- Then, you can fill out, modify, print, or sign the Nebraska Authorization to Use or Disclose Protected Health Information.
- Every legal template you obtain is yours indefinitely.
- To receive another copy of the bought form, navigate to the My documents section and click the corresponding button.
- If you are visiting the US Legal Forms site for the first time, follow the straightforward instructions below.
- Firstly, ensure that you have selected the correct template for the county/city of your choice.
- Read the form description to confirm you have chosen the correct document.
Form popularity
FAQ
Unauthorized access, use, and disclosure occur when protected health information is accessed or shared without the individuals' consent or outside the bounds defined by the Nebraska Authorization to Use or Disclose Protected Health Information. This can lead to significant privacy violations and potentially harmful consequences for individuals. Organizations must have strict policies and practices in place to prevent such unauthorized actions and ensure compliance with legal standards.
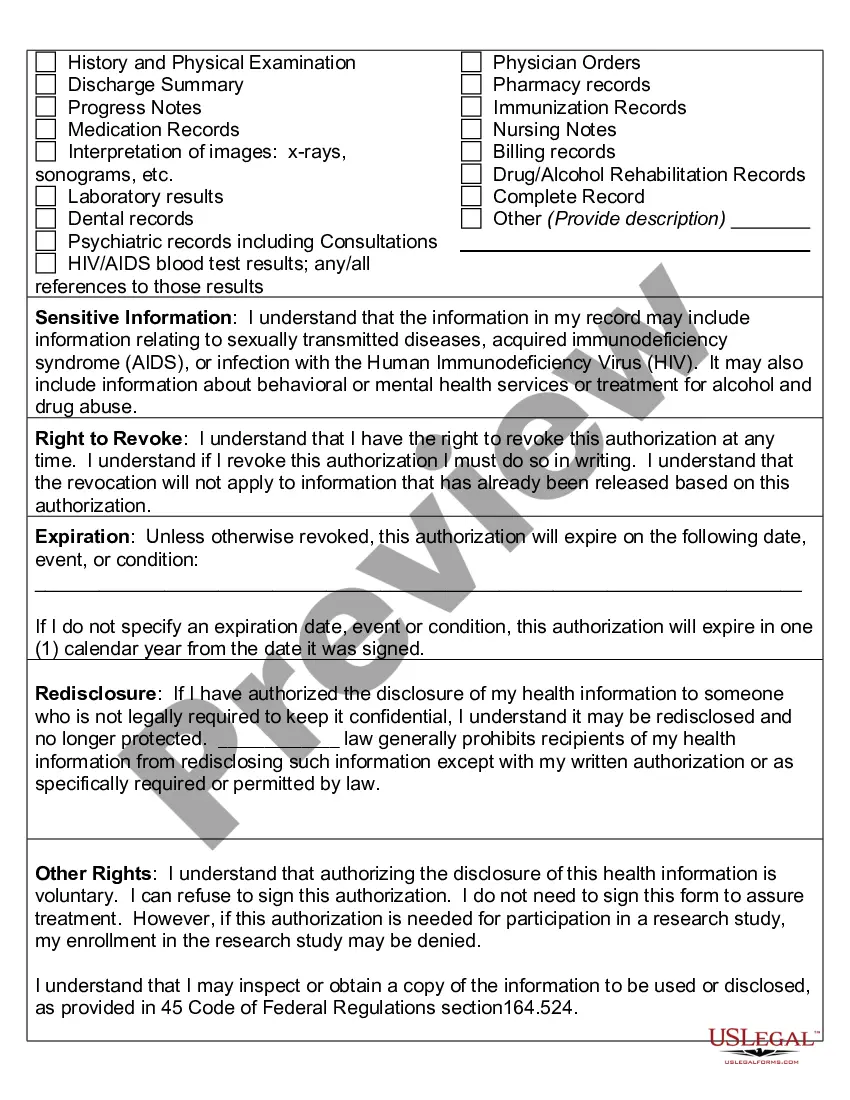
An authorization must specify a number of elements, including a description of the protected health information to be used and disclosed, the person authorized to make the use or disclosure, the person to whom the covered entity may make the disclosure, an expiration date, and, in some cases, the purpose for which the
The core elements of a valid authorization include:A meaningful description of the information to be disclosed.The name of the individual or the name of the person authorized to make the requested disclosure.The name or other identification of the recipient of the information.More items...
When is HIPAA Authorization Required? 45 CFR §164.508 details the uses and disclosures of PHI that require an authorization to be obtained from a patient/plan member before information can be shared or used. HIPAA authorization is required for: Use or disclosure of PHI otherwise not permitted by the HIPAA Privacy Rule.
A HIPAA authorization is a detailed document in which specific uses and disclosures of protected health are explained in full. By signing the authorization, an individual is giving consent to have their health information used or disclosed for the reasons stated on the authorization.
PHI concerning victims of abuse, neglect or domestic violence may be disclosed to a government authority, including social service or protective service agencies authorized to receive such reports. In these cases the disclosure must be required by law and limited to what the law allows.
A covered entity must obtain the individual's written authorization for any use or disclosure of protected health information that is not for treatment, payment or health care operations or otherwise permitted or required by the Privacy Rule.
When Must HIPAA Authorization be Obtained? The covered entity can use or disclosure of PHI for marketing purposes. If the marketing communication involves direct or indirect remuneration to the covered entity from a third party, the authorization must state that such remuneration is involved.
When Must HIPAA Authorization be Obtained? The covered entity can use or disclosure of PHI for marketing purposes. If the marketing communication involves direct or indirect remuneration to the covered entity from a third party, the authorization must state that such remuneration is involved.
You may disclose the PHI as long as you receive a request in writing. The written request must contain: the covered entity's name, the patient's name, the date of the event/time of treatment, and the reason for the request.