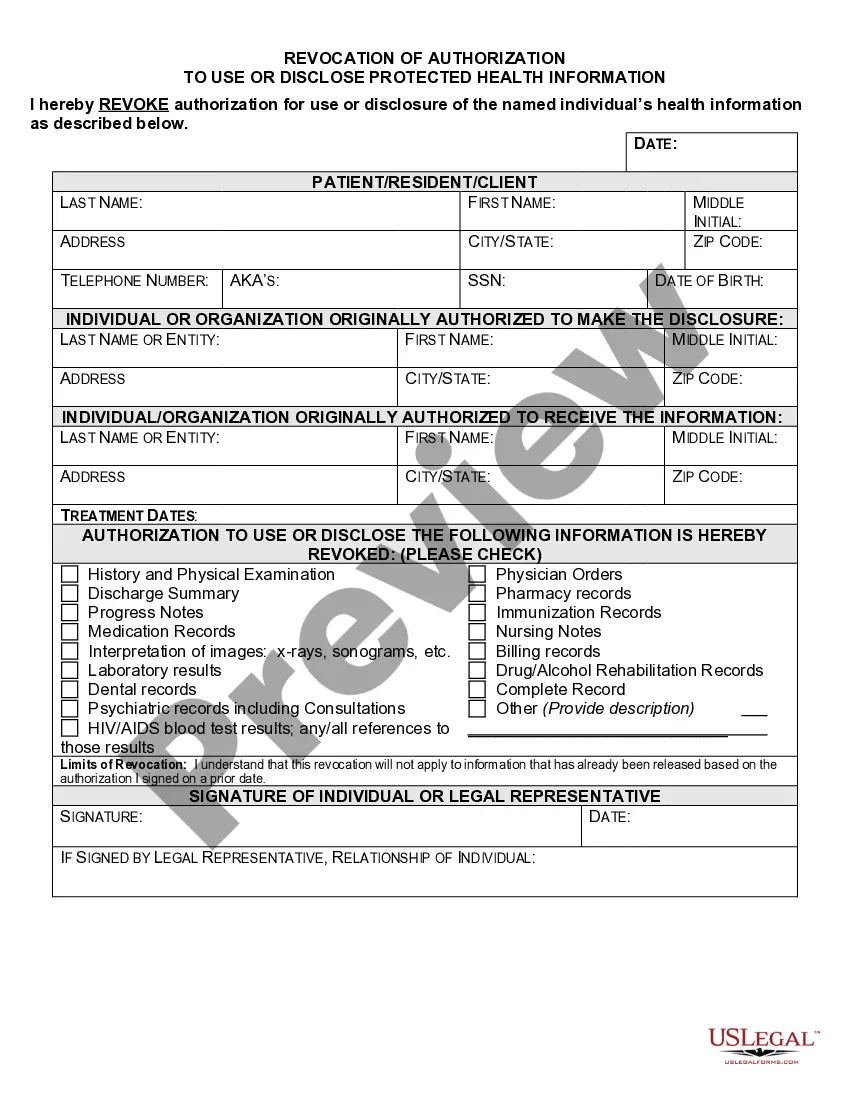
Connecticut Revocation of Authorization To Use or Disclose Protected Health Information
Description
How to fill out Revocation Of Authorization To Use Or Disclose Protected Health Information?
Are you currently in the situation where you require documents for either business or specific purposes almost daily? There are many legal document templates available online, but locating ones you can trust is not easy.
US Legal Forms provides numerous template options, such as the Connecticut Revocation of Authorization To Use or Disclose Protected Health Information, which are designed to meet state and federal regulations.
If you are already familiar with the US Legal Forms website and have an account, simply Log In. Then, you can download the Connecticut Revocation of Authorization To Use or Disclose Protected Health Information template.
- Obtain the form you require and verify it is for your specific city/region.
- Utilize the Review button to inspect the document.
- Check the description to ensure that you have selected the correct template.
- If the document is not what you’re looking for, use the Search field to find the one that meets your needs and requirements.
- Once you find the appropriate template, click Buy now.
- Choose the pricing plan you prefer, enter the necessary information to create your account, and complete the purchase using your PayPal or credit card.
- Select a convenient file format and download your copy.
Form popularity
FAQ
The HIPAA Privacy Rule requires that an individual provide signed authorization to a covered entity, before the entity may use or disclose certain protected health information (PHI).
Generally speaking, covered entities may disclose PHI to anyone a patient wants. They may also use or disclose PHI to notify a family member, personal representative, or someone responsible for the patient's care of the patient's location, general condition, or death.
Under the HIPAA Privacy Rule, a covered entity must disclose protected health information in only two situations: (a) to individuals (or their personal representatives) specifically when they request access to, or an accounting of disclosures of, their protected health information; and (b) to the Department of Health
A patient authorization is not required for disclosure of PHI between Covered Entities if the disclosure is needed for purposes of treatment or payment or for healthcare operations. You may disclose the PHI as long as you receive a request in writing.
What are two required elements of an authorization needed to disclose PHI? Response Feedback: All authorizations to disclose PHI must have an expiration date and provide an avenue for the patient to revoke his or her authorization. What does the term "Disclosure" mean?
A HIPAA authorization is a detailed document in which specific uses and disclosures of protected health are explained in full. By signing the authorization, an individual is giving consent to have their health information used or disclosed for the reasons stated on the authorization.
An authorization must specify a number of elements, including a description of the protected health information to be used and disclosed, the person authorized to make the use or disclosure, the person to whom the covered entity may make the disclosure, an expiration date, and, in some cases, the purpose for which the
Information can be shared without consent if it is justified in the public interest or required by law. Do not delay disclosing information to obtain consent if that might put children or young people at risk of significant harm.
Valid HIPAA Authorizations: A ChecklistNo Compound Authorizations. The authorization may not be combined with any other document such as a consent for treatment.Core Elements.Required Statements.Marketing or Sale of PHI.Completed in Full.Written in Plain Language.Give the Patient a Copy.Retain the Authorization.
Covered entities may use and disclose protected health information without individual authorization as required by law (including by statute, regulation, or court orders). Public Health Activities.