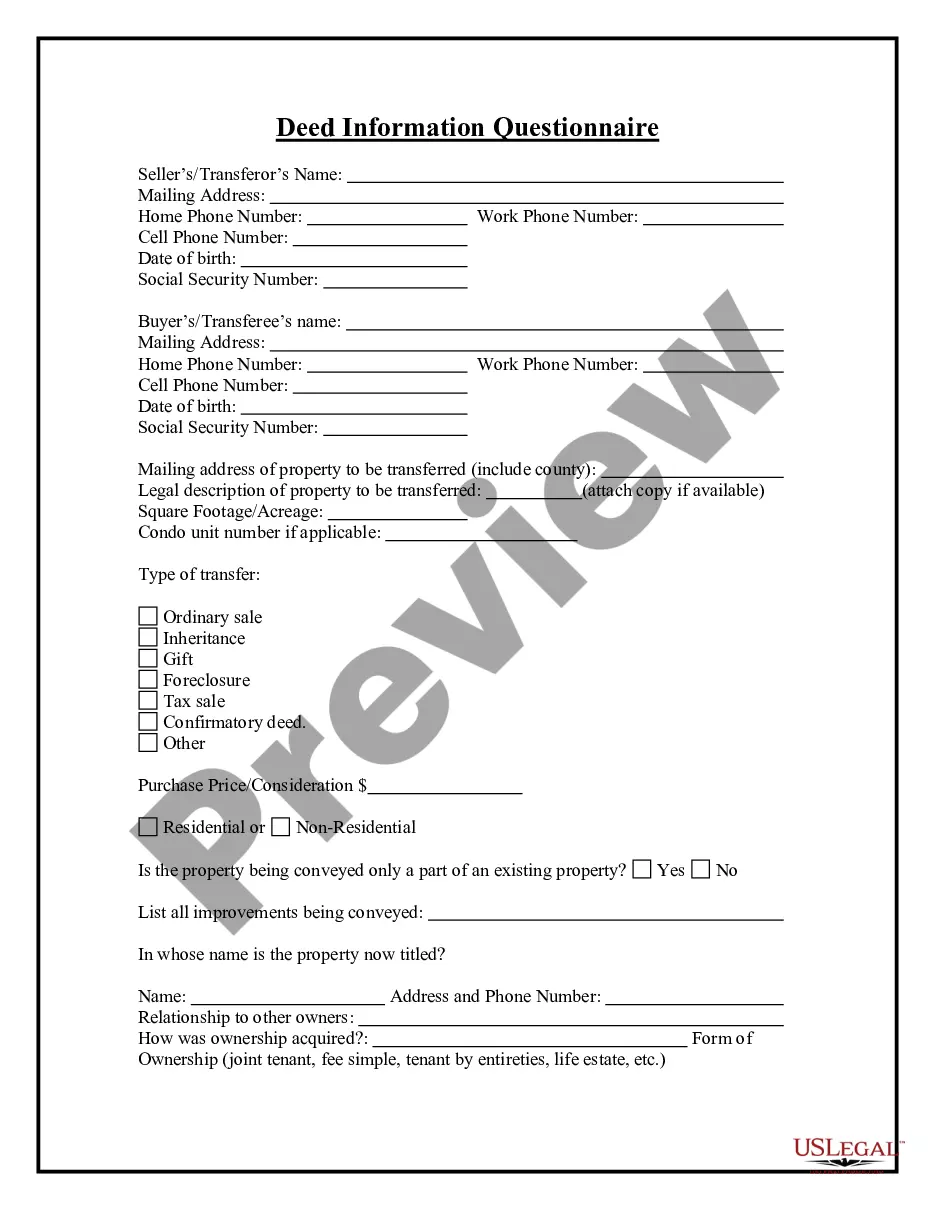
Transfer Death Form Blank With Blank Phosphates
Description
How to fill out Wisconsin Transfer On Death Or TOD To Beneficiary - Official Form Used To Record Beneficiary's Interest Following Death Of Grantor?
It’s well-known that you can’t instantly become a legal expert, nor can you comprehend how to swiftly draft the Transfer Death Form Blank With Blank Phosphates without possessing a tailored set of abilities.
Drafting legal documents is a lengthy process that necessitates specific training and expertise.
So why not entrust the preparation of the Transfer Death Form Blank With Blank Phosphates to the professionals.
You can restore access to your documents at any time from the My documents section. If you are an existing client, you can simply Log In and locate and download the template from the same section.
Regardless of the reason for your forms—whether financial, legal, or personal—our platform caters to your needs. Experience US Legal Forms today!
- Access the necessary form using the search feature located at the top of the webpage.
- View it (if this option is available) and review the accompanying description to determine if the Transfer Death Form Blank With Blank Phosphates meets your needs.
- Initiate your search again if you require a different document template.
- Create a free account and choose a subscription plan to purchase the form.
- Select Buy now. After the payment is processed, you can obtain the Transfer Death Form Blank With Blank Phosphates, fill it out, print it, and send or mail it to the appropriate parties or organizations.
Form popularity
FAQ
In a phosphate transfer reaction, a phosphate group is transferred from a phosphate group donor molecule to a phosphate group acceptor molecule.
When the plant or animal dies, it decays, and the organic phosphate is returned to the soil. Within the soil, organic forms of phosphate can be made available to plants by bacteria that break down organic matter to inorganic forms of phosphorus. This process is known as mineralisation.
Phosphates exist in three forms: orthophosphate, metaphosphate (or polyphosphate) and organically-bound phosphate; each compound contains phosphorous in a different chemical arrangement.
The chemical formula of Phosphate is PO43-. Phosphate contains one Phosphorus (P) atom and four Oxygen (O) atoms. In which one central Phosphorus (P) atom is surrounded by four Oxygen (O) atoms. It forms an ionic bond between these 2 atoms.
In serum, phosphate exists in two forms, dihydrogen phosphate (H2PO4) and its salt, mono-hydrogen phosphate (HPO4). The relationship between these two can be determined by the Henderson-Hasselbalch equation. At the physiologic pH of 7.40, the pK of H2PO4 is 6.8 and the ratio of HPO4 to H2PO4 is .