Warning Notice Forensic Medicine
Description
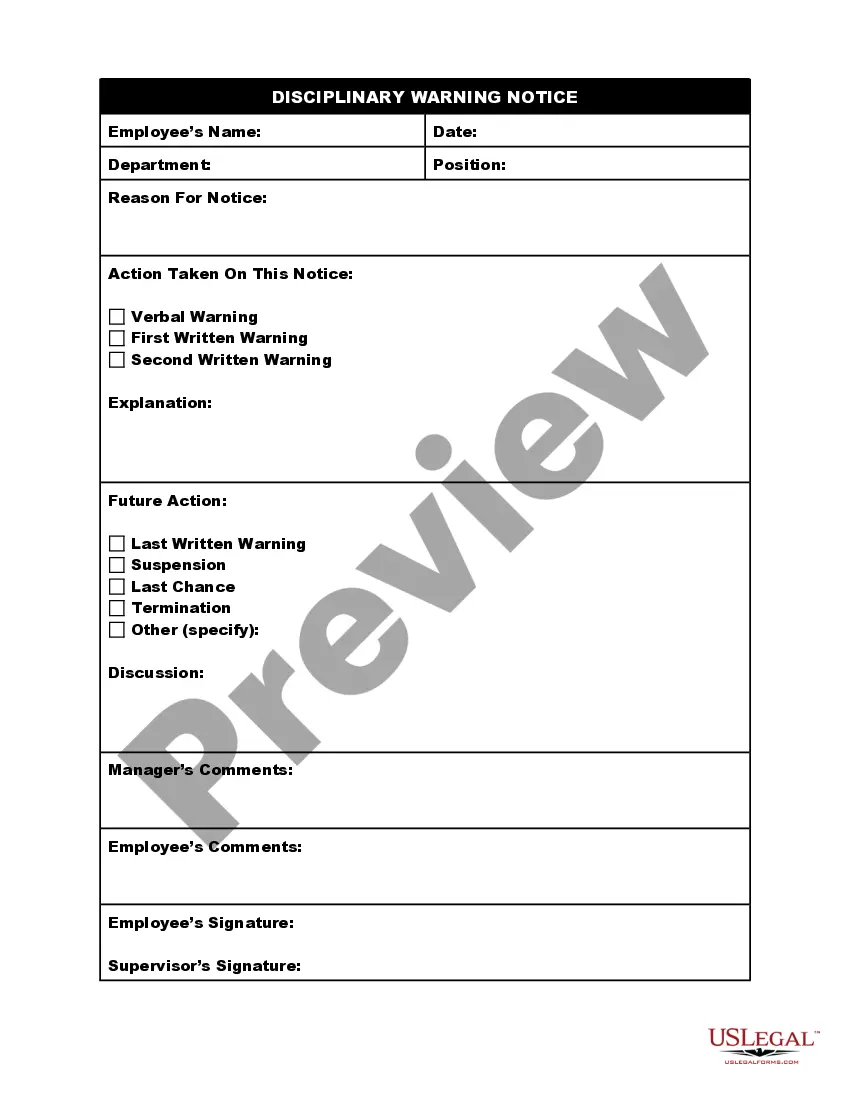
How to fill out Employee Warning Notice?
Identifying a preferred location to obtain the most up-to-date and suitable legal templates is part of the challenge in navigating bureaucracy.
Finding the correct legal documents requires accuracy and meticulousness, which is why it is essential to source Warning Notice Forensic Medicine samples exclusively from reliable providers, such as US Legal Forms.
Eliminate the stress associated with your legal paperwork. Explore the vast US Legal Forms database to discover legal templates, check their suitability for your needs, and download them right away.
- Utilize the catalog navigation or search bar to find your template.
- Check the description of the form to determine if it meets the criteria of your state and locality.
- Examine the form preview, if available, to ensure that the template is indeed what you are looking for.
- If the Warning Notice Forensic Medicine does not match your requirements, continue searching for the appropriate document.
- Once you are confident in the form’s applicability, download it.
- If you are a logged-in user, click Log in to verify your identity and access your chosen forms in My documents.
- If you haven’t created an account, click Buy now to acquire the template.
- Choose the pricing plan that fits your needs.
- Proceed with registration to complete your purchase.
- Complete your transaction by selecting a payment method (credit card or PayPal).
- Select the file format for downloading the Warning Notice Forensic Medicine.
- After obtaining the form on your device, you can modify it using the editor or print it out and complete it by hand.
Form popularity
FAQ
A Warning Letter from the FDA signifies that the agency has identified violations of laws or regulations. It outlines specific issues that need immediate attention and provides a timeframe for compliance. This letter serves as a critical step in regulatory enforcement and highlights the importance of maintaining high standards in practices related to forensic medicine.
If you ignore an FDA warning letter, the situation can escalate quickly. The FDA may impose additional fines, increase inspections, or take legal action against the company. Moreover, continued non-compliance can result in restrictions on the marketability of products. Therefore, addressing the letter promptly is vital to protect your business in warning notice forensic medicine.
The consequences of a Warning Letter can vary but often lead to substantial operational change. Affected parties may experience heightened scrutiny from regulators, loss of business, and potential lawsuits. Additionally, it can strain relationships with clients and stakeholders. Staying informed and proactive is key in the landscape of warning notice forensic medicine.
An FDA warning letter is a serious matter that indicates a breach of regulatory compliance. It serves as a formal notification that significant issues require immediate action. Ignoring it can lead to more severe penalties, including product seizures or facility shutdowns. Thus, addressing the concerns raised is essential for any entity involved in forensic medicine.
Consequences of FDA warning letters can be quite significant. Organizations may face increased inspections, potential legal repercussions, and damage to their public reputation. Compliance often requires immediate corrective actions, which can be resource-intensive. This emphasizes the importance of legal preparedness in warning notice forensic medicine.
After receiving a Warning Letter from the FDA, the organization must take immediate action to rectify the outlined issues. This could include a detailed response plan addressing the FDA's concerns. It’s crucial to document all changes made, as failure to comply may lead to further legal implications. Understanding this process is vital in the field of warning notice forensic medicine.
Yes, FDA warning letters are public documents, accessible to anyone interested in the compliance history of a company. This transparency allows consumers and professionals in forensic medicine to make informed decisions about the organizations they engage with. Consequently, maintaining compliance can safeguard a facility’s reputation and credibility in the industry.
Several factors can trigger an FDA warning letter, including repeated violations, failure to correct issues identified in previous inspections, and significant health risks to the public. Forensic medicine facilities must maintain rigorous compliance to avoid such notifications. Being proactive in addressing any findings can mitigate the risk of receiving a warning letter.
The main difference between a 483 and a warning letter is the severity of the compliance issues they address. A 483 identifies specific observations that need attention, while a warning letter signifies more serious infractions that could lead to enforcement actions. Understanding this distinction is important for professionals in forensic medicine, as addressing 483 issues may prevent a warning letter.
An FDA warning letter is a formal notice issued by the FDA to inform a company of significant violations related to its products or practices. This letter can lead to legal action if the issues are not resolved within a specified timeframe. In the realm of forensic medicine, receiving a warning letter can impact the reputation of a facility, making it vital to take swift corrective actions.