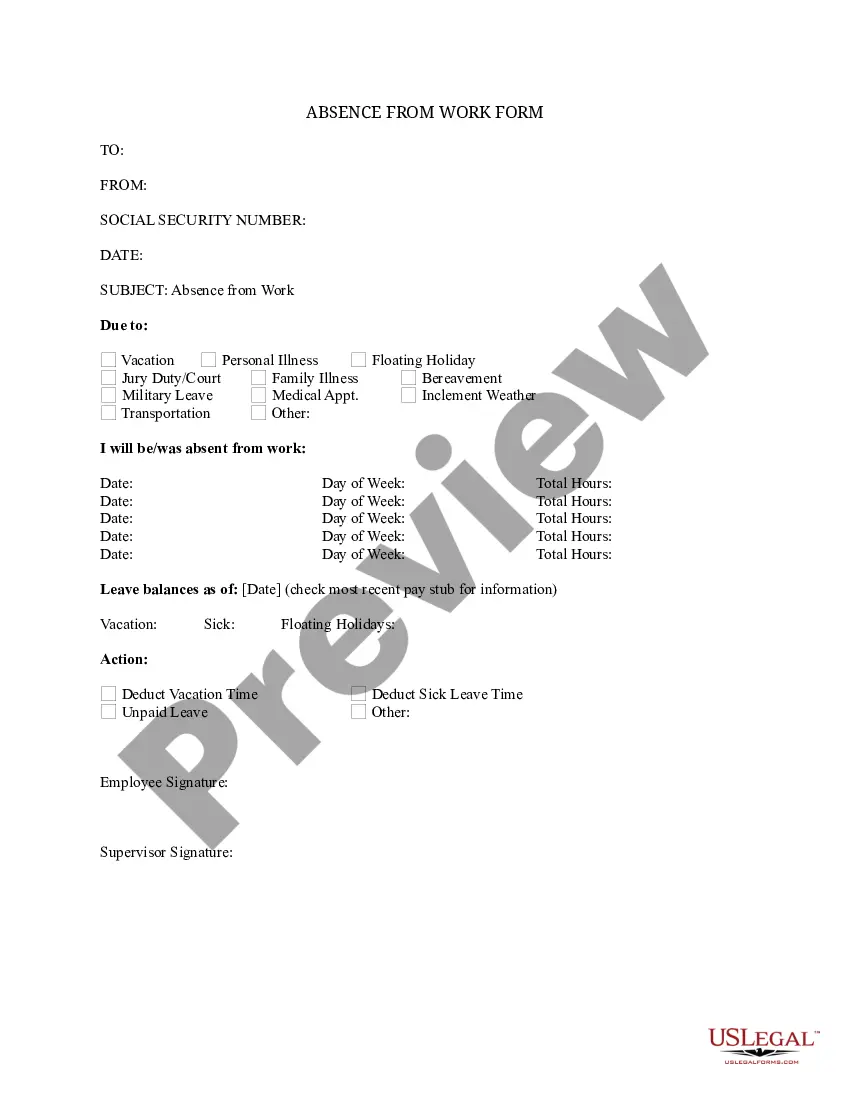
Sample Consent Letter For Research Study
Description
How to fill out Sample Letter Explaining The Purposes Of The Waiver And Consent Regarding An Estate?
When you need to complete a Sample Consent Letter For Research Study in accordance with your local state's laws and guidelines, there may be numerous options to choose from.
There's no necessity to scrutinize every form to ensure it fulfills all the legal requirements if you are a US Legal Forms subscriber.
It is a dependable resource that can assist you in obtaining a reusable and current template on any topic.
Utilize the Preview mode and examine the form description if available.
- US Legal Forms is the most extensive online catalog with a repository of over 85,000 ready-to-use documents for business and personal legal situations.
- All templates are verified to comply with each state's laws and regulations.
- Therefore, when downloading a Sample Consent Letter For Research Study from our platform, you can be confident that you retain a legitimate and updated document.
- Accessing the necessary sample from our platform is remarkably straightforward.
- If you already possess an account, simply Log In to the system, ensure your subscription is active, and save the selected file.
- Later, you can navigate to the My documents tab in your profile and access the Sample Consent Letter For Research Study anytime.
- If this is your inaugural experience with our website, please follow the guide outlined below.
- Browse the proposed page and verify it for alignment with your criteria.
Form popularity
FAQ
Filling out a consent form involves carefully reading all the information in the Sample consent letter for research study. You should address any questions or concerns before signing, ensuring you fully understand what participation entails. Typically, you will need to provide your name, date, and signature, verifying that you consent to participate under the outlined terms. Platforms like US Legal Forms offer templates that guide you through this process, making it straightforward and efficient.
A simple example of consent can be found in a Sample consent letter for research study. This document clearly outlines the study's purpose, procedures, and potential risks while ensuring participants understand their rights. It serves as an agreement that the person voluntarily agrees to participate. By signing, individuals indicate that they have read the letter and believe they can make informed decisions about their involvement.
An example of a consent statement includes a clear declaration from participants about their understanding of the study and their choice to participate. A standard form may state, 'I have read and understood the information provided, and I consent to participate in this research study.' Such statements are essential in providing participants with assurance that they are making informed choices. Reviewing a sample consent letter for research study can provide context for crafting effective consent statements.
The five elements of consent are competence, comprehension, voluntariness, disclosure, and agreement. Participants must have the mental capacity to understand the information, comprehend what they are agreeing to, and voluntarily choose to participate without coercion. Each element reinforces the ethical foundation of the study and protects participant rights. For guidance, consider a sample consent letter for research study that illustrates these elements.
To write a consent form for a research study, start by outlining the study’s purpose and procedures, then detail potential risks and benefits. Ensure you use clear language and provide ample space for questions from participants. It’s beneficial to review existing templates, such as a sample consent letter for research study, which can serve as a framework while crafting your unique document.
The five steps of consent include providing information, allowing participants time to think, answering questions, obtaining the participant's agreement, and documenting the consent. Each step is crucial for ensuring that participants fully understand the study before agreeing to participate. Following these steps promotes ethical research practices and builds participant confidence. Reviewing a sample consent letter for research study can help illustrate these steps clearly.
A consent form must contain the study's title, the researcher’s contact information, details about what participation involves, important risks, and how confidentiality will be maintained. This information is critical for participants to understand what they are agreeing to. Transparency in communication prepares participants for their involvement and helps protect their rights. For reference, you can check a sample consent letter for research study.
The five key points to include in a consent form are the purpose of the study, the procedures participants will undergo, potential risks and benefits, confidentiality measures, and the right to withdraw. Each of these points provides essential information for participants to make an informed decision. Ensuring clarity in these areas builds trust and ensures ethical standards. Look for a sample consent letter for research study to exemplify how to present this information effectively.
An example of informed consent for a study is a document that explains the research, the procedures involved, and the rights of the participants. It clearly outlines any potential risks and benefits associated with participation. The document should ensure participants understand their role and have the right to withdraw at any time. You can find a sample consent letter for research study to help guide you in creating an effective consent document.
To write a consent letter, begin by clearly stating the purpose of the letter and whom it concerns. Include details about the research, such as its objectives and procedures, to ensure complete understanding. It’s important to conclude with a section for signatures, confirming the informed consent of the participants, which can greatly benefit from using a well-structured sample consent letter for research study available on platforms like US Legal Forms.