Death Form Blank With Blank Phosphates
Description
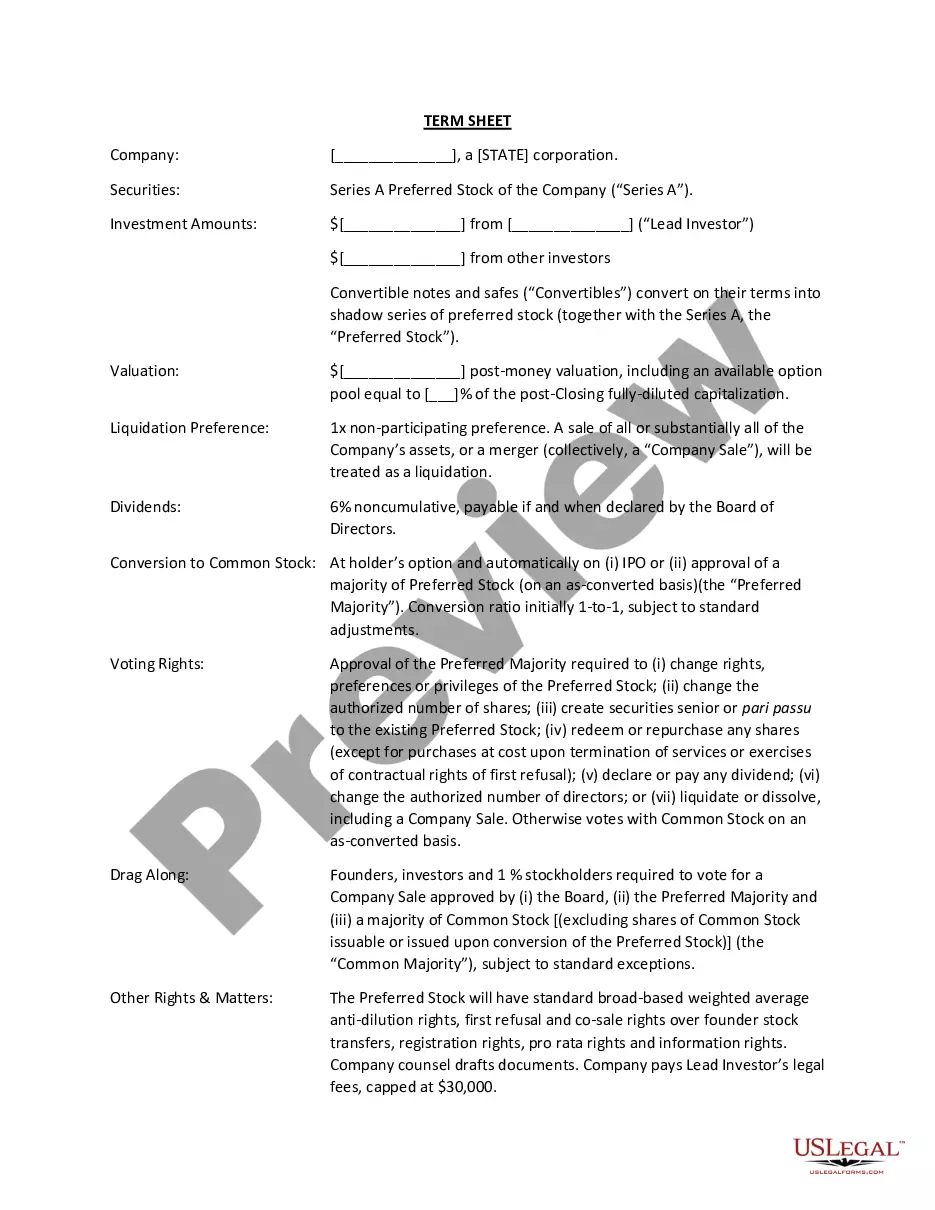
How to fill out Affidavit Of Death Of Joint Tenant?
It’s obvious that you can’t become a legal professional immediately, nor can you learn how to quickly draft Death Form Blank With Blank Phosphates without the need of a specialized background. Creating legal forms is a long venture requiring a specific training and skills. So why not leave the creation of the Death Form Blank With Blank Phosphates to the specialists?
With US Legal Forms, one of the most comprehensive legal document libraries, you can find anything from court paperwork to templates for internal corporate communication. We understand how crucial compliance and adherence to federal and local laws are. That’s why, on our platform, all templates are location specific and up to date.
Here’s start off with our website and obtain the form you need in mere minutes:
- Find the form you need with the search bar at the top of the page.
- Preview it (if this option available) and check the supporting description to figure out whether Death Form Blank With Blank Phosphates is what you’re searching for.
- Start your search again if you need any other template.
- Set up a free account and choose a subscription option to buy the form.
- Pick Buy now. Once the transaction is through, you can get the Death Form Blank With Blank Phosphates, complete it, print it, and send or send it by post to the designated people or organizations.
You can re-gain access to your forms from the My Forms tab at any time. If you’re an existing client, you can simply log in, and locate and download the template from the same tab.
No matter the purpose of your paperwork-be it financial and legal, or personal-our website has you covered. Try US Legal Forms now!
Form popularity
FAQ
Phosphorus cycles through plants and animals much faster than it does through rocks and sediments. When animals and plants die, phosphates will return to the soils or oceans again during decay. After that, phosphorus will end up in sediments or rock formations again, remaining there for millions of years.
Together with nitrogen, arsenic, antimony, and bismuth, phosphorus is classified as a pnictogen. Phosphorus is an element essential to sustaining life largely through phosphates, compounds containing the phosphate ion, PO43?.
Particularly common in granitic pegmatites are the primary phosphates apatite [Ca5(F,Cl,OH)(PO4)3], triphylite [LiFePO4], lithiophilite [LiMnPO4], and the rare-earth phosphates monazite [(LaCe)(PO4)] and xenotime [Y(PO4)].
Organic phosphates are commonly found in the form of esters as nucleotides (e.g. AMP, ADP, and ATP) and in DNA and RNA.
Phosphates are compounds made of phosphorus and oxygen. The most common base phosphate unit is PO4. In simplified terms, phosphates can be derived as esters or salts of phosphoric acid.