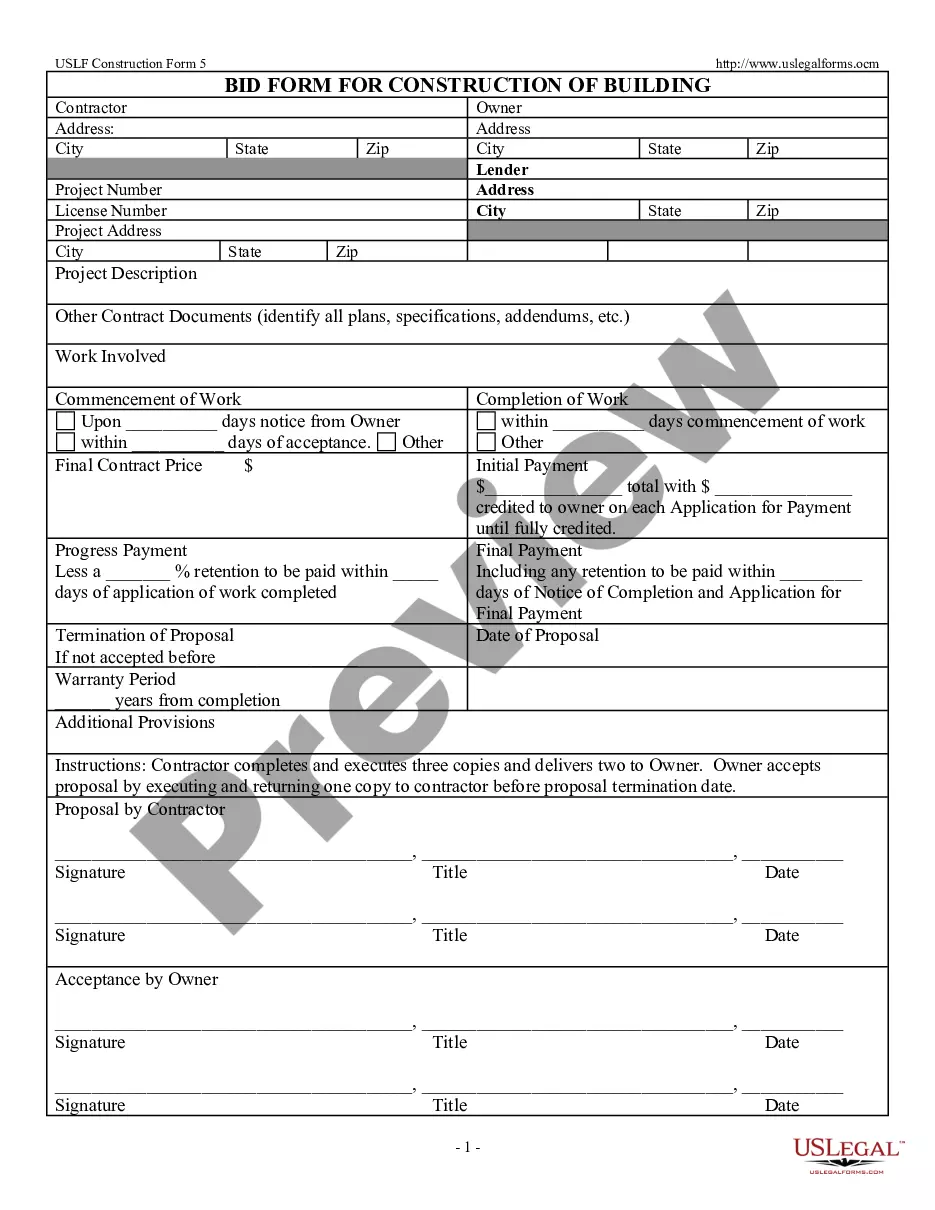
Order Setting Bond With Bond Length
Description
How to fill out Order Setting Bond?
Finding a reliable source for the latest and most applicable legal templates is a significant part of grappling with red tape. Identifying the appropriate legal documents requires accuracy and meticulousness, which is why it is crucial to obtain samples of Order Setting Bond With Bond Length solely from reputable sources, such as US Legal Forms. An incorrect template can squander your time and delay the matter at hand. With US Legal Forms, you have minimal concerns. You can access and review all the details regarding the document’s applicability and significance for your situation and in your jurisdiction.
Consider the following steps to complete your Order Setting Bond With Bond Length.
Eliminate the hassle associated with your legal paperwork. Browse the extensive US Legal Forms library to discover legal templates, assess their relevance to your situation, and download them instantly.
- Utilize the library navigation or search bar to find your template.
- Examine the form’s description to determine if it meets the criteria of your jurisdiction.
- Check the form preview, if available, to confirm the template is what you need.
- Return to the search and seek the appropriate template if the Order Setting Bond With Bond Length does not meet your requirements.
- If you are confident about the form’s applicability, download it.
- As an authorized user, click Log in to verify and access your selected forms in My documents.
- If you do not yet have an account, click Buy now to acquire the template.
- Choose the pricing option that fits your needs.
- Proceed to the registration to complete your purchase.
- Conclude your purchase by selecting a payment method (credit card or PayPal).
- Choose the document format for downloading Order Setting Bond With Bond Length.
- Once you have the form on your device, you can modify it with the editor or print it and fill it out manually.
Form popularity
FAQ
Hence, the correct order of bond lengths is C?C>C?H>C=C>C?C.
Bond length can be calculated in three easy steps. First, determine the type of covalent bond between the atoms (single, double or triple). Then, using a covalent radii chart, find the atomic radii in these bonds. Finally, add them together and you have the approximate bond length.
The length of the bond is determined by the number of bonded electrons (the bond order). The higher the bond order, the stronger the pull between the two atoms and the shorter the bond length.
To calculate bond length, one must draw the Lewis structure for the molecule, find the atomic radii of the two atoms on an atomic radius chart, and add the two atomic radii together. For example, the bond length of H2 is approximately 62pm, since both atoms are hydrogen atoms and their covalent radius is 31pm.
Bond order (B.O.) is defined as one-half the difference between the number of electrons present in the bonding and the anti-bonding orbitals. Bond order (B.O) = ( Number of bonding electrons - Number of the anti-bonding electron) Bond order = (Nb- Na)