Home Fee Cost Plus Contract With Gmp In Franklin
Category:
State:
Multi-State
County:
Franklin
Control #:
US-00462
Format:
Word;
Rich Text
Instant download
Description
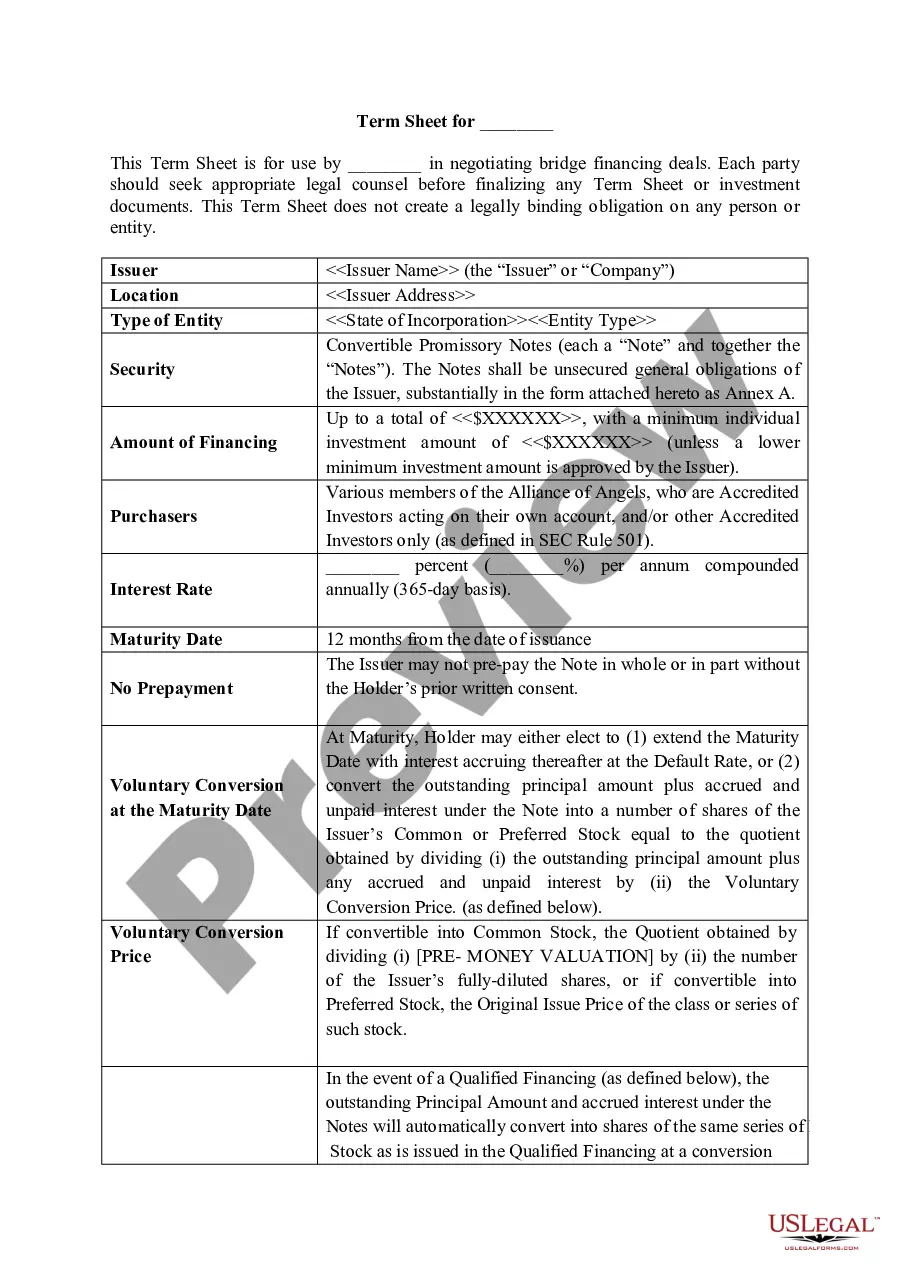
The Home Fee Cost Plus Contract with GMP in Franklin is a comprehensive construction agreement that outlines the responsibilities and expectations between a contractor and an owner. Key features include the scope of work, work site details, permit requirements, and insurance obligations. The contract specifically allows for a cost-plus arrangement where the owner agrees to pay the actual costs incurred by the contractor plus a predefined fee for services. It also addresses potential changes in the scope of work, late payment fees, and warranty limitations. Filling and editing instructions emphasize the need for owners to specify project details and any changes through written change orders to avoid misunderstandings. This document is particularly useful for attorneys, partners, owners, associates, paralegals, and legal assistants who seek clarity in construction agreements, ensuring compliance with legal standards while protecting their interests. By utilizing this contract, users can facilitate a smoother communication process throughout the construction project, mitigate legal risks, and ensure all parties are held accountable.
Free preview
Form popularity
FAQ
Good manufacturing practice ( GMP ) is the minimum standard that a medicines manufacturer must meet in their production processes. Products must: be of consistent high quality. be appropriate to their intended use. meet the requirements of the marketing authorisation ( MA ) or product specification.