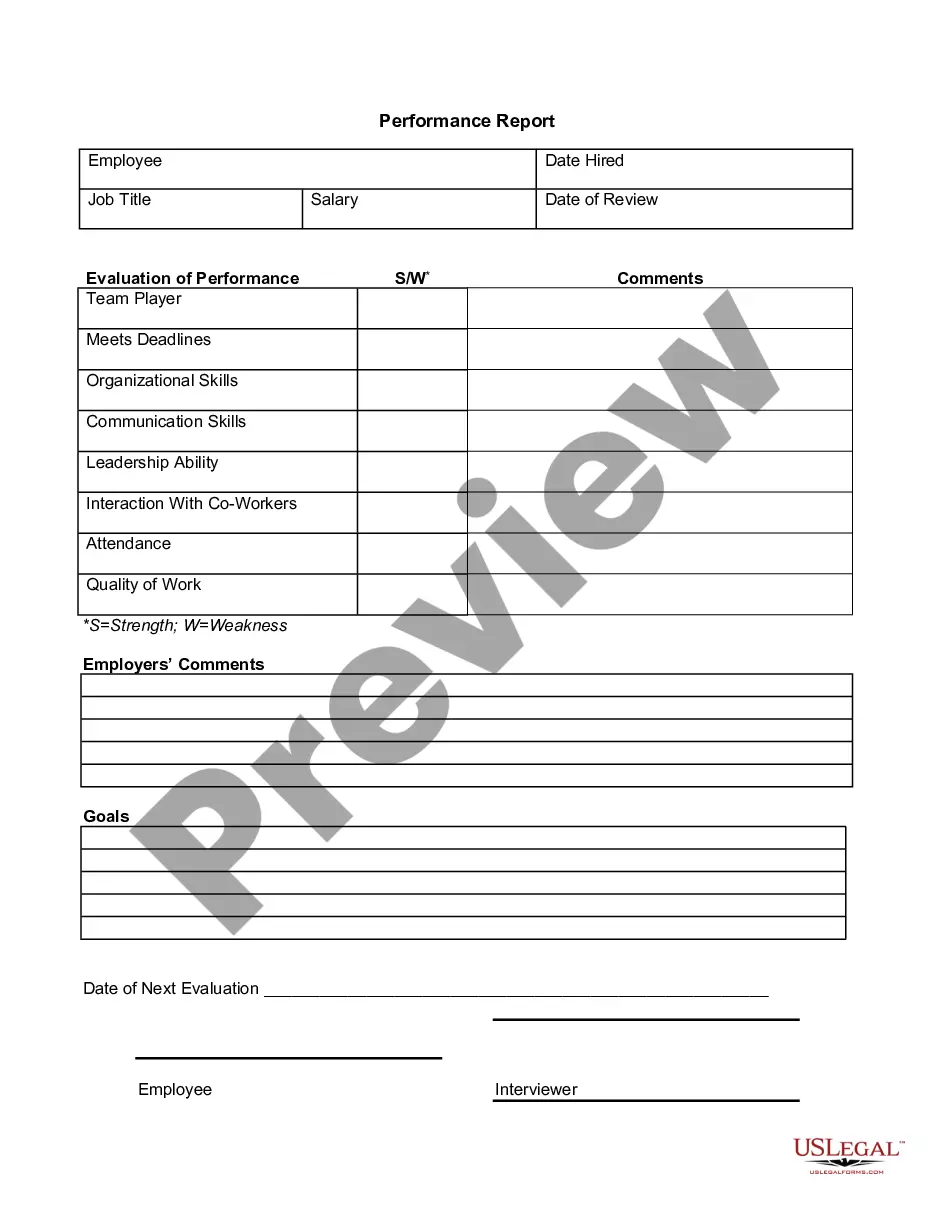
Consent Release Information Form Psychologist In Utah
Description
Form popularity
FAQ
The example of informed consent is when a patient is advised by an EMT of the risks associated with refusing care, ensuring the patient's decision is well-informed. Informed consent is a fundamental ethical and legal principle in healthcare, involving full disclosure of treatment risks and benefits to the patient.
Informed consent documents A statement that the project is research and participation is voluntary, A summary of the research, including: Purpose. Duration. List of procedures. Reasonable, foreseeable risks or discomforts. Reasonable, expected benefits. Alternative procedures or course of treatment, if any.
Instructions for Developing an Informed Consent Document General Information. Describe the purpose(s) of this research study in lay terms. Purpose of the Study. Procedures. Risks. Benefits. Compensation, Costs and Reimbursement. Withdrawal or Termination from Study. Confidentiality.
Examples of giving verbal consent include: “Yes” “That sounds great” “That feels awesome”
Basic Elements of Informed Consent Purpose of the Research. Description of the Research. Risks. Benefits. Alternatives to Participation.
I understand the information provided for the study insert title as described herein. My questions have been answered to my satisfaction, and I agree to participate in this study. I have been given a copy of this form.
I participant name, agree to participate or agree to participation of my child participant name in the research project titled project title, conducted by researcher(s) name who has (have) discussed the research project with me. I have received, read and kept a copy of the information letter/plain language statement.
Consent to treatment means a person must give permission before they receive any type of medical treatment, test or examination. This must be done on the basis of an explanation by a clinician. Consent from a patient is needed regardless of the procedure, whether it's a physical examination or something else.
At a minimum, a well-designed informed consent form will address the following information: Risks and benefits of treatment. Fees and payment policies. Confidentiality and its limits. Contact information and communication. Social media policy and general boundaries. Emergency procedures.
A document with important information about a medical procedure or treatment, a clinical trial, or genetic testing. It also includes information on possible risks and benefits. If a person chooses to take part in the treatment, procedure, trial, or testing, he or she signs the form to give official consent.