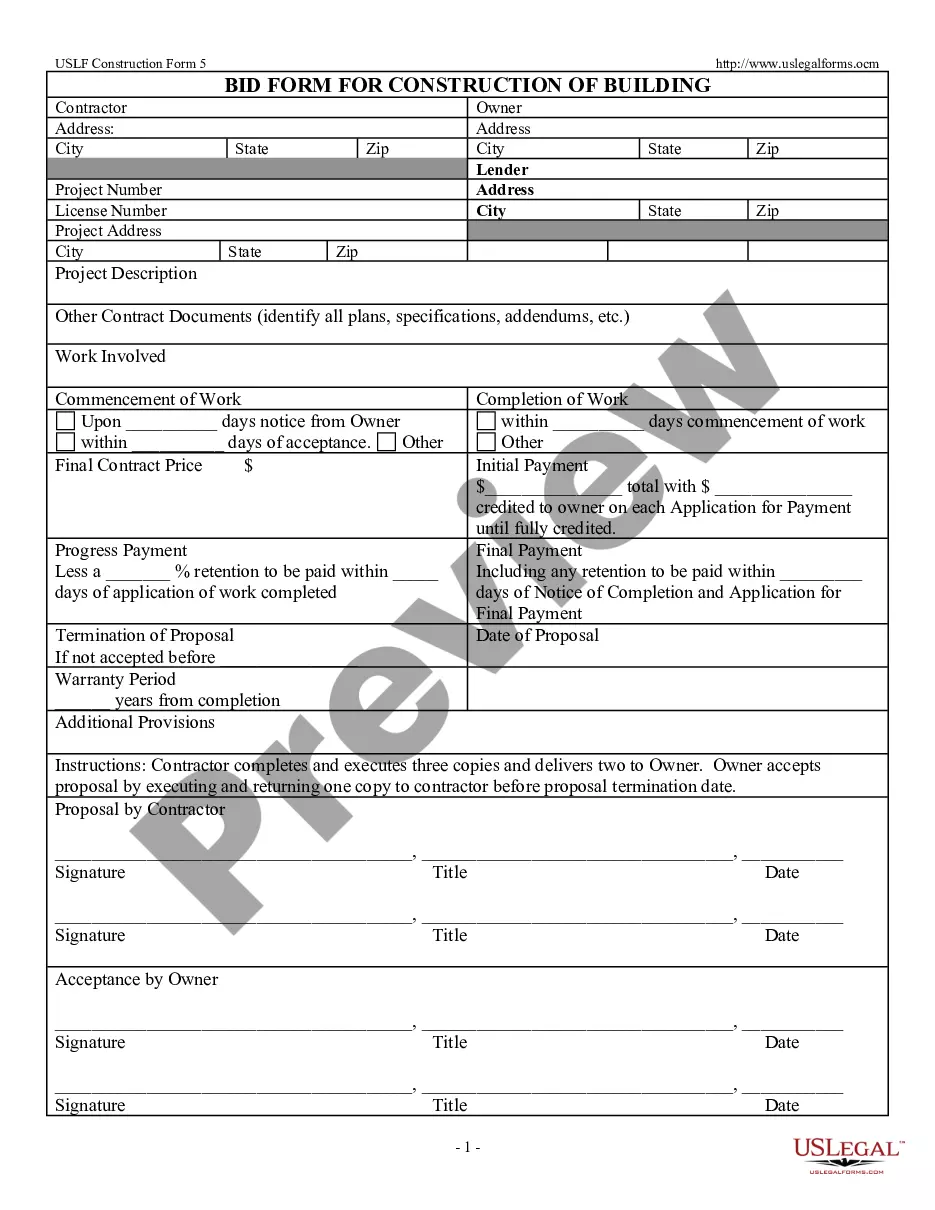
Employee Consent Form Template In Franklin
Description
Form popularity
FAQ
How to Write a Consent Letter Title the Letter: Start with a clear title, such as "Consent Letter" or "Permission Letter." Include Your Contact Information: At the top of the letter, include your name, address, phone number, and email address. Date the Letter: Write the date the letter is being written.
How to write a consent form: A step-by-step guide Step 1: Title and introduction. Step 2: Description of the activity. Step 3: Risks and benefits. Step 4: Confidentiality and data handling. Step 5: Voluntary participation and withdrawal. Step 6: Consent statement. Step 7: Signature and date. Step 8: Contact information.
Instructions for Developing an Informed Consent Document General Information. Describe the purpose(s) of this research study in lay terms. Purpose of the Study. Procedures. Risks. Benefits. Compensation, Costs and Reimbursement. Withdrawal or Termination from Study. Confidentiality.
All sections of the consent form, except the "Consent" section, should be written in second person ("You are invited..."). Headers should include “Informed Consent” followed by the title of the study (e.g., the header in this document). Footers should include page numbers.
Language - Consent forms should be written in the 2nd person (i.e., "you are") and in a language that is clear, concise, and understandable to the subject population. This includes both reading level and language (e.g, English, Spanish, French).
Informed consent language should be written in the second person (“you”), not in the first person (“I”). Minimize passive voice to the extent possible. Example of passive voice: “A summary of results will be sent to all study participants.” Example of active voice: “We will send you a summary of the results.”
How to write a consent form: A step-by-step guide Step 1: Title and introduction. Step 2: Description of the activity. Step 3: Risks and benefits. Step 4: Confidentiality and data handling. Step 5: Voluntary participation and withdrawal. Step 6: Consent statement. Step 7: Signature and date. Step 8: Contact information.
The entire informed consent process involves giving a subject adequate information concerning the study, providing adequate opportunity for the subject to consider all options, responding to the subject's questions, ensuring that the subject has comprehended this information, obtaining the subject's voluntary agreement ...