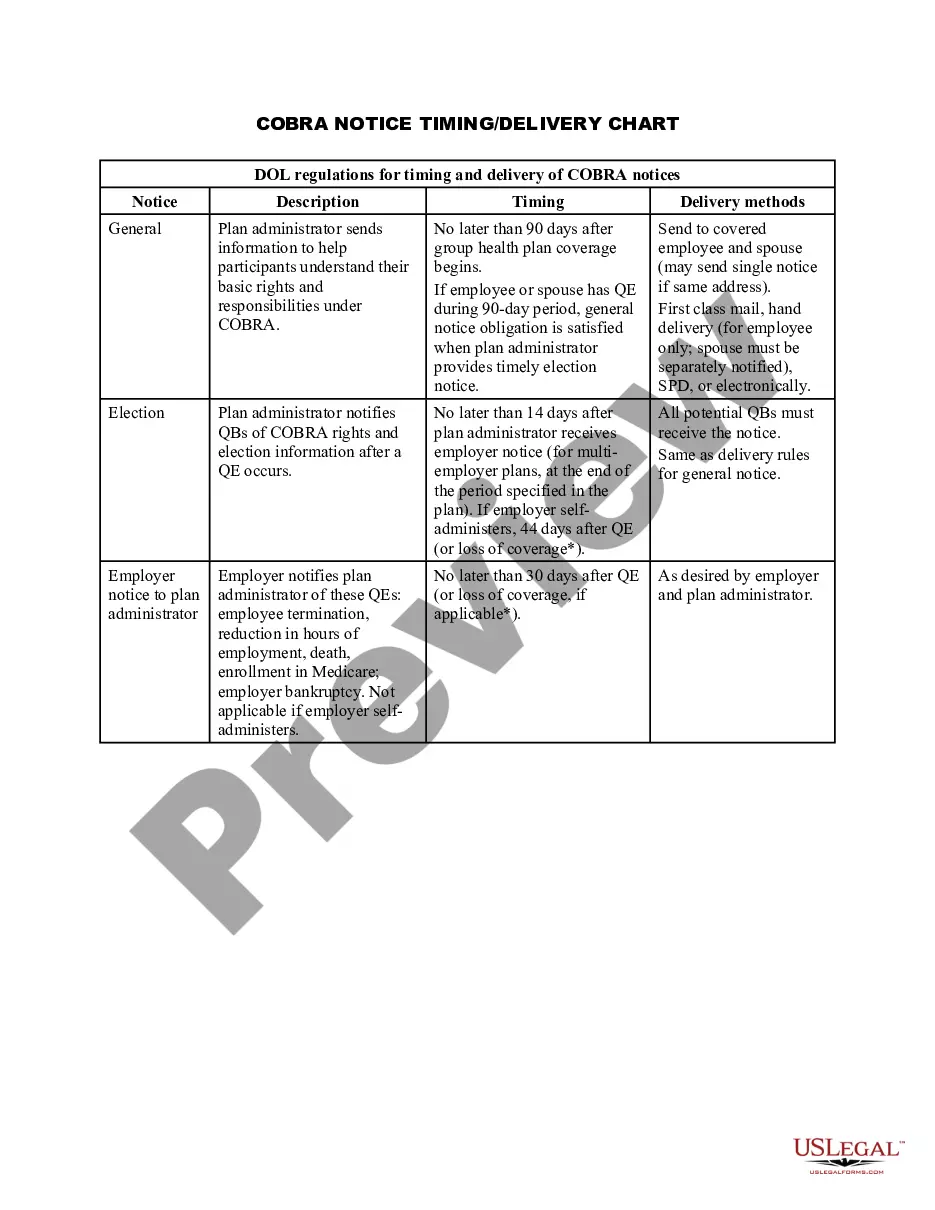
Waiver Of Consent Example
Description
How to fill out Mississippi Waiver Of Process And General Entry Of Appearance And Joinder In Complaint?
The Waiver Of Consent Template displayed on this page is a reusable professional document created by experienced attorneys in compliance with federal and local statutes and regulations.
For over 25 years, US Legal Forms has supplied individuals, businesses, and legal professionals with more than 85,000 validated, state-specific documents for every business and personal circumstance. It’s the fastest, simplest, and most dependable method to acquire the necessary paperwork, as the service ensures bank-level data protection and anti-malware safeguards.
Register for US Legal Forms to access verified legal templates for all of life’s situations at your fingertips.
- Search for the document you need and examine it.
- Choose the pricing plan that suits you and create an account.
- Select the format you prefer for your Waiver Of Consent Template (PDF, DOCX, RTF) and store the sample on your device.
- Print the template to complete it by hand or use an online multifunctional PDF editor to quickly and accurately fill out and sign your form with a legally-binding electronic signature.
- Reaccess the same document whenever necessary by visiting the My documents tab in your profile to redownload any previously saved documents.
Form popularity
FAQ
Hear this out loud PauseWaiver of documentation of consent: Also known as a verbal consent or waiving a signed consent. The investigator obtains consent, and the consent process still has all the requirements as written consent, but the subject does not sign a consent form.
Hear this out loud PauseWaiver of All Consent In certain cases, federal regulations allow the IRB to waive the requirement to obtain any informed consent. Most complete waivers of consent involve studies in which there are minimal risks to subjects, but complete waivers are also possible in emergency care and other limited circumstances.
For research that is FDA-regulated, a waiver of documentation of consent may be granted only: When the research presents no more than minimal risk of harm to participants and involves no procedures for which written consent is normally required outside the research context.
Hear this out loud PauseThe Common Rule allows an IRB to approve a consent procedure that does not include, or that alters, some or all of the elements of informed consent (or to waive the requirement altogether) if it finds that the research falls into either of the following two categories: The first category involves research that must be ...
Hear this out loud PauseWrite directly to the reader, as though you are explaining the facts in person. Informed consent language should be written in the second person (?you?), not in the first person (?I?). Minimize passive voice to the extent possible.