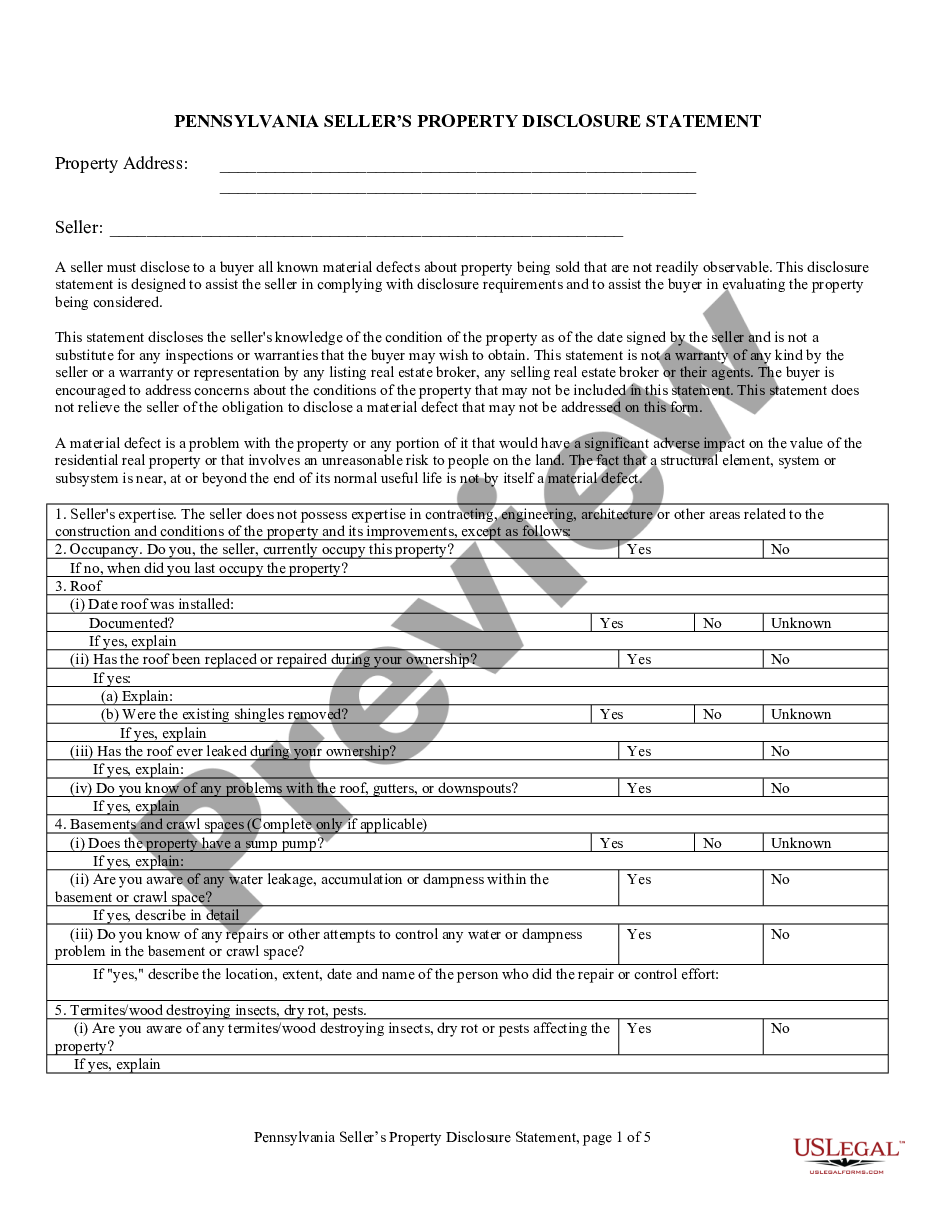
New Mexico Agreement with Sponsor for Research on New Product or Process
Description
How to fill out Agreement With Sponsor For Research On New Product Or Process?
Selecting the appropriate legal document template can be a challenge.
Clearly, there is an abundance of templates available online, but how do you find the legal form you require.
Utilize the US Legal Forms website. The service offers thousands of templates, such as the New Mexico Agreement with Sponsor for Research on New Product or Process, suitable for both business and personal use.
First, confirm you've chosen the correct form for your city/state. You can review the form using the Preview button and check the form details to ensure it is the right one for you.
- All forms are reviewed by professionals and comply with state and federal regulations.
- If you are already registered, Log In to your account and click on the Obtain button to download the New Mexico Agreement with Sponsor for Research on New Product or Process.
- Use your account to browse the legal forms you have purchased previously.
- Go to the My documents section of your account and obtain another copy of the document you need.
- If you are a new user of US Legal Forms, here are simple steps to follow.
Form popularity
FAQ
Subject's Rights, Safety, and Bell-Being. ICH GCP Principle 3 states that the rights, safety, and well-being of the trial subjects are the most important considerations and should prevail over interests of science and society.
Investigator Sponsored Studies are defined as unsolicited research originating from an external sponsor entity, institution or organization and include studies also known as investigator sponsored trials (IST), expert initiated research (EIR) or any other term which may reference investigator-sponsored or investigator-
Nonrefundable advance payments for future clinical trial management services should initially be capitalized and then expensed as the related services are performed. Company A should continue to evaluate whether it expects the services to be rendered.
Data Integrity and System Validation The new guidance identifies data integrity as a cornerstone of GCP, and states that trial data systems must ensure data integrity, particularly with regard to technology changes and software updates.
Industry Sponsored Clinical Trial: A human subjects research study whose primary purpose is to assess the safety and/or efficacy in humans of a drug, device, diagnostic, treatment, preventive measure, or similar intervention through testing of the intervention on patients in a clinical setting.
Good Clinical Practice (GCP) is an international ethical and scientific quality standard for designing, conducting, recording and reporting trials that involve the participation of human subjects.
The objective of this ICH GCP Guideline is to provide a unified standard for the European Union (EU), Japan and the United States to facilitate the mutual acceptance of clinical data by the regulatory authorities in these jurisdictions.
A primary purpose of the ICH is to: Minimize the need for redundant research. The ICH GCP Guidelines: Set standards for the design, conduct, monitoring and reporting of clinical research.
All research requires a sponsor. The sponsor is the individual, company, institution or organisation that takes on legal responsibility for the initiation, management and/or financing of the research.
Industry-sponsored research is paid for by an industry organization that has contracted with a faculty member to conduct a clinical trial that involves an intervention with, or observation of, a disease or biomedical condition, or a registry/repository related to a disease or biomedical condition.