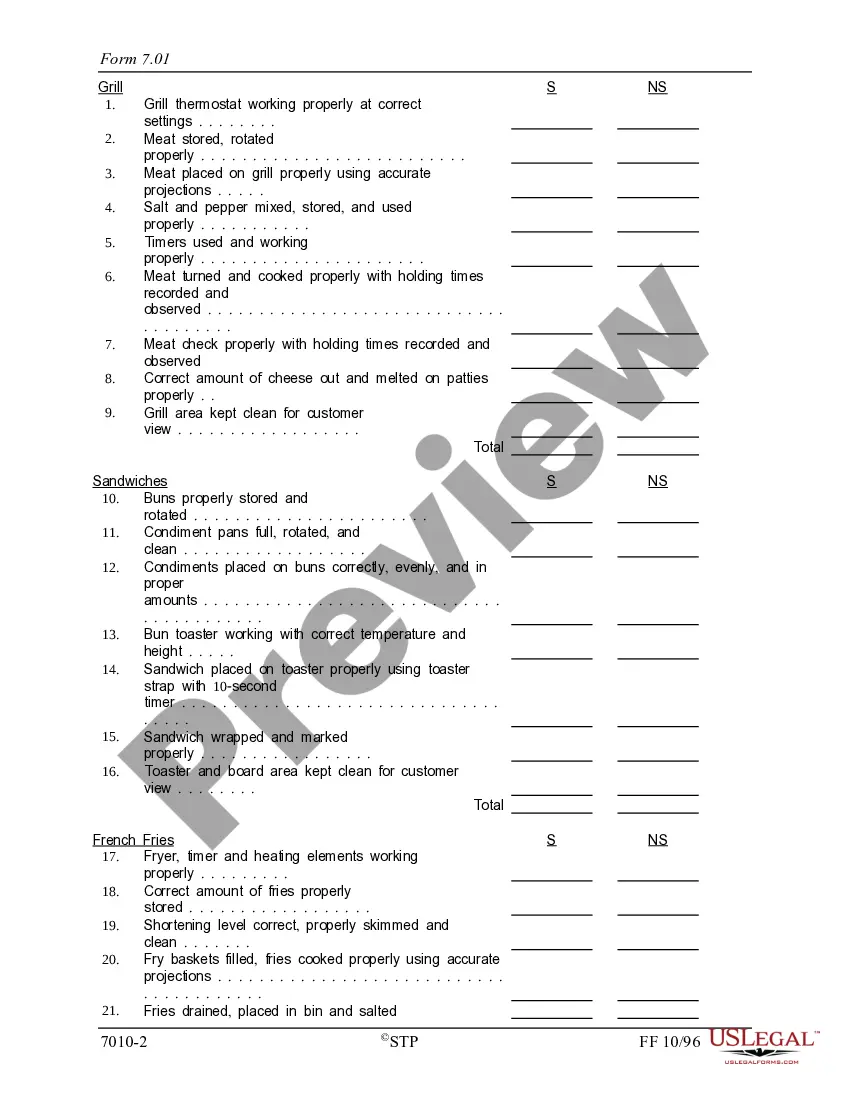
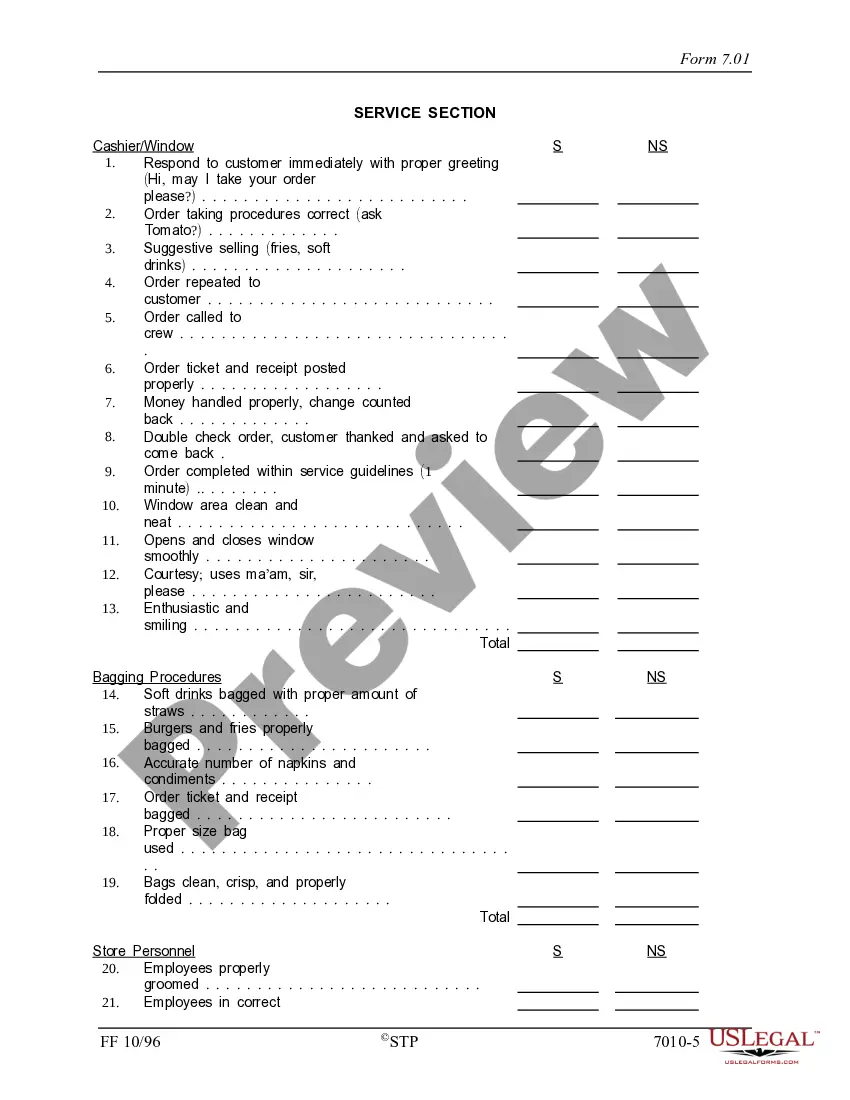
Georgia Quality Assurance Report
Description
How to fill out Quality Assurance Report?
If you have to total, obtain, or produce legitimate papers layouts, use US Legal Forms, the largest selection of legitimate types, that can be found on the Internet. Use the site`s easy and convenient look for to get the paperwork you require. Different layouts for company and personal uses are categorized by groups and suggests, or search phrases. Use US Legal Forms to get the Georgia Quality Assurance Report in a couple of clicks.
When you are previously a US Legal Forms buyer, log in in your profile and then click the Down load option to find the Georgia Quality Assurance Report. Also you can accessibility types you in the past downloaded inside the My Forms tab of your own profile.
If you use US Legal Forms for the first time, refer to the instructions beneath:
- Step 1. Make sure you have chosen the shape for that appropriate town/country.
- Step 2. Make use of the Review solution to check out the form`s content material. Don`t forget to learn the explanation.
- Step 3. When you are not satisfied using the type, take advantage of the Look for area at the top of the screen to get other models in the legitimate type format.
- Step 4. Upon having identified the shape you require, click on the Purchase now option. Pick the prices prepare you choose and add your credentials to register to have an profile.
- Step 5. Process the purchase. You may use your bank card or PayPal profile to accomplish the purchase.
- Step 6. Select the format in the legitimate type and obtain it on your gadget.
- Step 7. Full, change and produce or signal the Georgia Quality Assurance Report.
Each legitimate papers format you acquire is your own property permanently. You have acces to every single type you downloaded within your acccount. Select the My Forms section and choose a type to produce or obtain again.
Be competitive and obtain, and produce the Georgia Quality Assurance Report with US Legal Forms. There are millions of expert and condition-particular types you can use for your personal company or personal requires.
Form popularity
FAQ
For example, quality assurance needs to check if the labeling of a food product lists all its ingredients and allergy warnings to ensure customer safety. If problems do occur, it's up to the quality assurance teams to find the causes for the breach in food safety and fix the issues to prevent them from happening again.
Quality assurance is a dynamic process through which nurses assume accountability for quality. of care they provide. It is a guarantee to the society that services provided by nurses are being. regulated by members of profession.
Quality Assurance Plans: Recommended Practices Identify data quality objectives for your data or project. Identify requirements for. ... Describe a structure for data storage that can also facilitate checking for errors and help to document data quality. Describe approved data entry tools and procedures, when applicable.
Quality assurance (QA) is any systematic process of determining whether a product or service meets specified requirements. QA establishes and maintains set requirements for developing or manufacturing reliable products.
How to write QA documentation General information about the project. A list of checks to be run. The type of verification being used (positive or negative). Available test data. Brief explanation of how the system is expected to behave. Details about the test environment (e.g. which browser version is being used).
The four types of quality assurance are pre-production inspection (PPI), during production inspection (DPI), pre-shipment inspection (PSI), and container loading/loading supervision (LS).
Quality issues can frequently arise in manufacturing, and quality assurers monitor and help fix these problems before they cause damage. For example, if a company manufactures shoes and a quality assurance team discovers the soles are coming loose in recent batches, they may start an investigation into the issue.
Define your business's standards and objectives. Envision what you want your customer support/service team to accomplish. ... Assign roles and responsibilities. ... Implement the new QA policies and procedures. ... Analyze and measure progress. ... Make adjustments as needed.
In order to write a status report, it is important to mention the following points: Summary of testing activities performed; Metrics on test case execution and defect rates; Overview of open and resolved defects; Description of any testing challenges or issues encountered;
Ing to the Health Resources and Services Administration (HRSA), quality assurance (QA) measures compliance against certain necessary standards, typically focusing on individuals, whereas quality improvement (QI) is a continuous improvement process focused on processes and systems.