Confidentiality Statement For Survey
Description
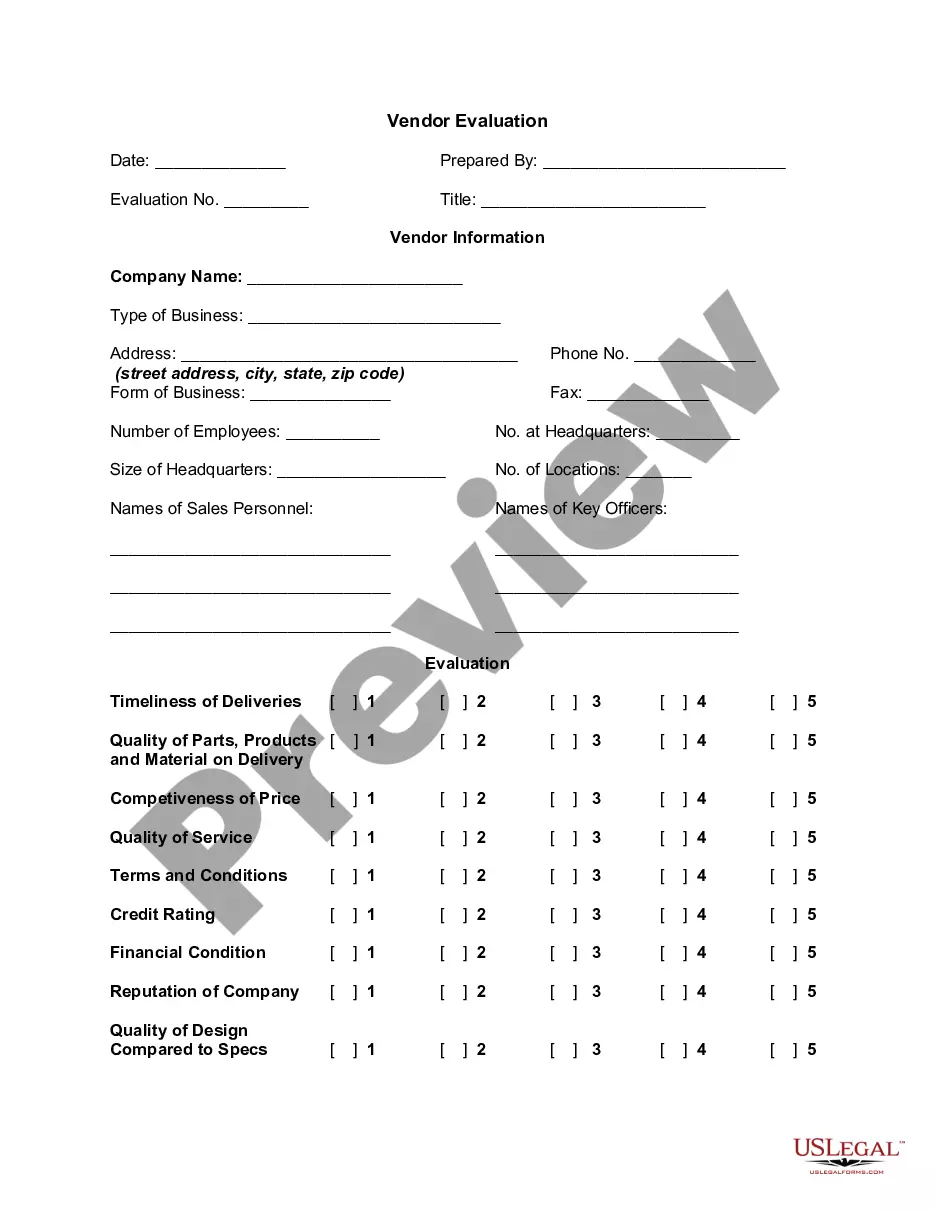
How to fill out Confidentiality Statement And Agreement For An Employee?
When you are required to finalize a Confidentiality Declaration For Survey that aligns with your local state's statutes and guidelines, there may be numerous choices to select from.
There's no requirement to verify each form to confirm it fulfills all the legal criteria if you are a US Legal Forms subscriber.
It is a reliable service that can assist you in obtaining a reusable and current template on any topic.
Using the Preview mode and reviewing the form description if available.
- US Legal Forms is the most extensive online repository with a compilation of over 85k ready-to-use documents for business and personal legal matters.
- All templates are verified to conform to each state's regulations.
- Thus, when downloading Confidentiality Declaration For Survey from our platform, you can be assured that you possess a valid and current document.
- Retrieving the required sample from our platform is straightforward.
- If you already have an account, simply Log In to the system, verify that your subscription is active, and save the selected file.
- In the future, you can access the My documents tab in your profile and gain entry to the Confidentiality Declaration For Survey at any time.
- If this is your first encounter with our library, please follow the instructions below.
- Browse through the suggested page and verify it against your criteria.
Form popularity
FAQ
1. keep all the research information shared with me confidential. I will not discuss or share the research information with anyone other than with the Researcher(s) or others identified by the Researcher(s). 2.
State that you have no intention of using survey response information other than for your questionnaire. Be upfront about the confidentiality in a very clear and easy to understand manner, rather than providing a huge disclaimer in legal jargon that nobody will read.
Do I always have to get informed consent? A. Yes, though in some circumstances IRBs can waive the requirement for this to be a written document. The informed consent process is a basic ethical obligation for researchers.
A confidentiality statement, also called a confidentiality agreement or clause or a non-disclosure agreement (NDA), is a binding contract. The other party agrees to keep certain information to themselves, and not disclose it. In other words, the other party must keep that information a secret.
Methods for keeping data confidential range from using routine precautions, such as substituting codes for participant identifiers and storing data in locked cabinets, to more elaborate procedures involving statistical methods (e.g., error inoculation) or data encryption.