Ga Disclosure Statement For Medical Device Security
Description
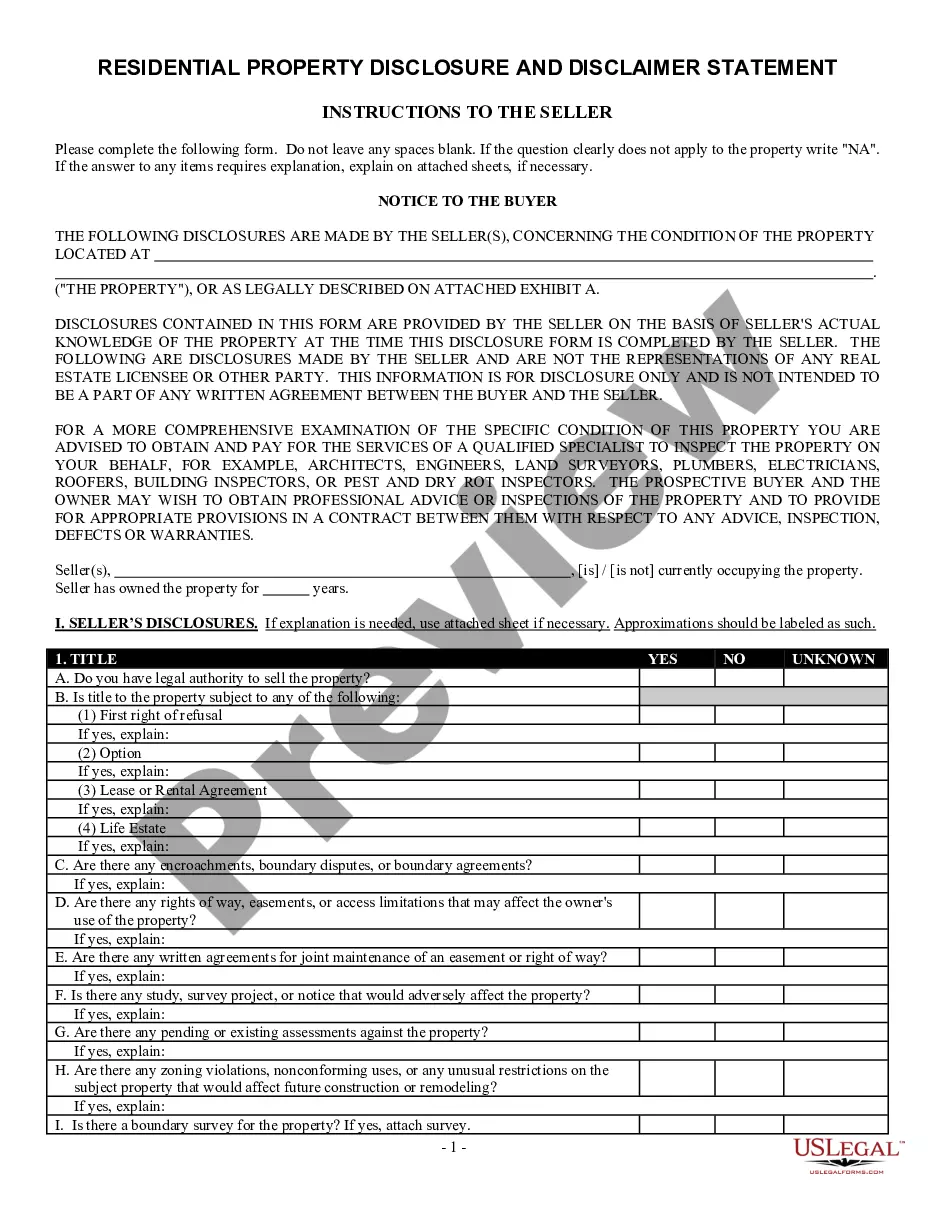
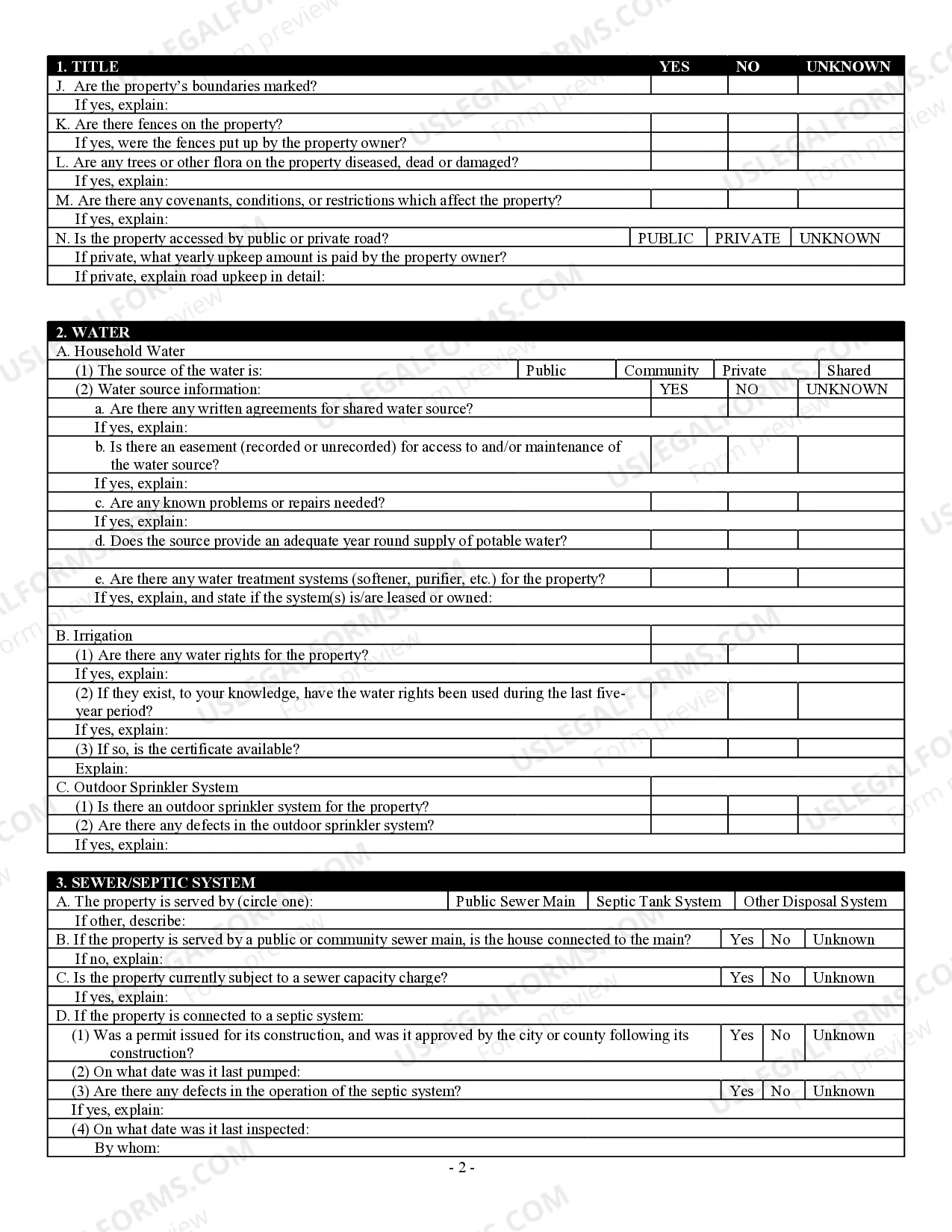
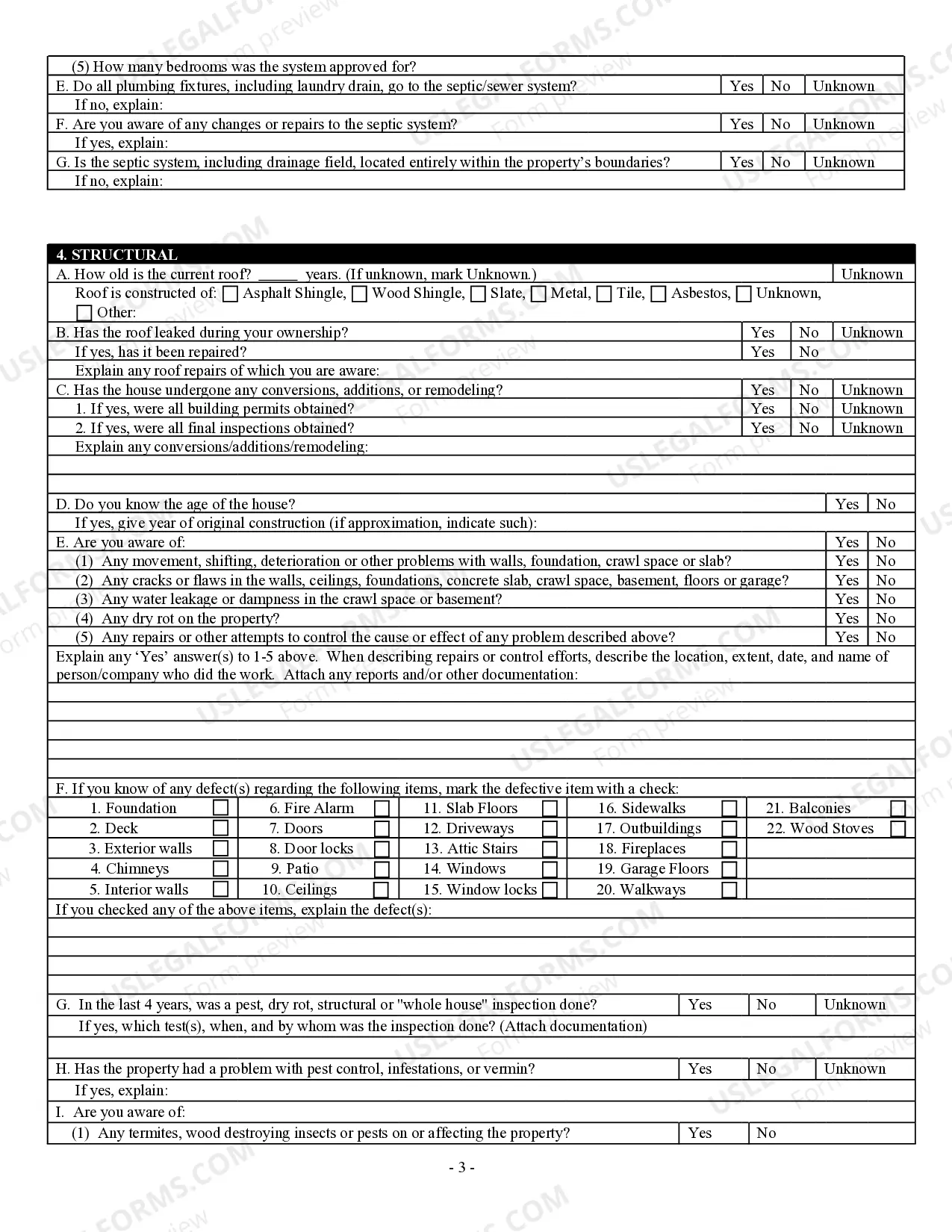
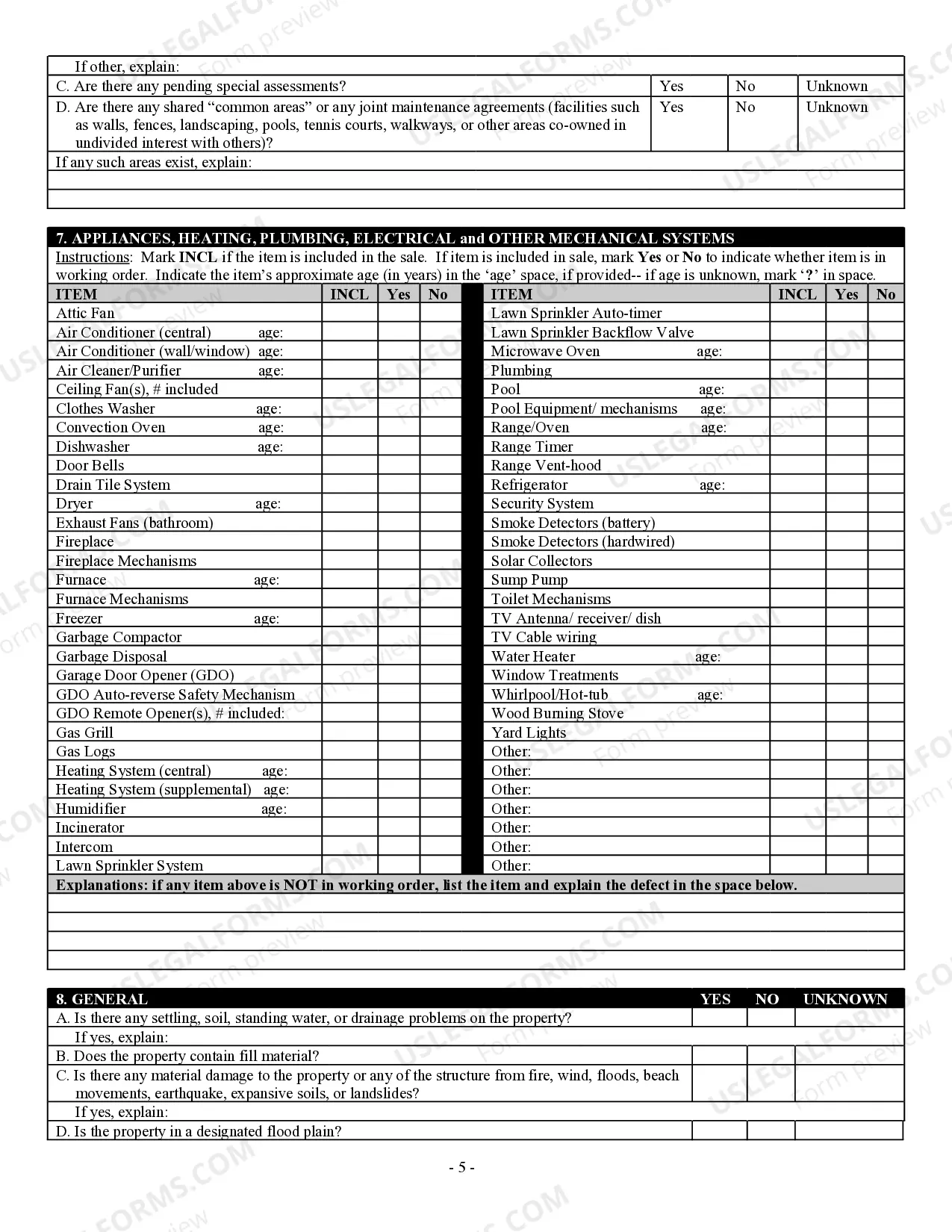
How to fill out Georgia Residential Real Estate Sales Disclosure Statement?
It’s widely known that you cannot instantly become a legal authority, nor can you swiftly learn how to prepare a Ga Disclosure Statement For Medical Device Security without possessing a specialized background.
Drafting legal documents is a lengthy procedure that demands specific education and expertise. So why not entrust the creation of the Ga Disclosure Statement For Medical Device Security to the experts.
With US Legal Forms, one of the largest legal document collections, you can discover anything from court forms to templates for in-office correspondence. We recognize how crucial compliance and adherence to federal and state laws and regulations are.
Establish a free account and choose a subscription plan to purchase the template.
Select Buy now. Once the payment is finalized, you can acquire the Ga Disclosure Statement For Medical Device Security, complete it, print it, and send or mail it to the required individuals or organizations.
- That’s why, on our platform, all templates are location-specific and current.
- Here’s how you can begin with our website and obtain the document you require in just minutes.
- Locate the form you need using the search bar at the top of the page.
- Preview it (if this option is available) and read the accompanying description to determine if the Ga Disclosure Statement For Medical Device Security is what you are looking for.
- If you need any other template, restart your search.
Form popularity
FAQ
The manufacturer disclosure statement for medical device security is a document that highlights the security measures and potential risks associated with a medical device. This statement serves to inform users and healthcare professionals about how the device protects patient data and maintains integrity. Utilizing resources like USLegalForms can help you create a comprehensive GA disclosure statement for medical device security, ensuring you cover all necessary aspects.
The MDS2 standard refers to a specific guideline that manufacturers follow to disclose pertinent information about medical device security. This standard ensures that manufacturers provide essential details about device vulnerabilities and security measures. Adhering to the MDS2 standard is vital for fulfilling the GA disclosure statement for medical device security, as it enhances trust and safety in medical devices.
Yes, the MDS2 form, which stands for Manufacturer Disclosure Statement, is often required for many medical devices. This document provides detailed information regarding a device's security features and protocols. If you are navigating the complexities of the GA disclosure statement for medical device security, having the MDS2 helps ensure compliance and promotes transparency in medical device usage.
The manufacturer disclosure statement for medical devices outlines essential information about a device's safety, effectiveness, and compliance with regulations. This statement helps healthcare providers and users understand the product better. When dealing with the GA disclosure statement for medical device security, this information is crucial for ensuring that all security measures are clearly communicated and documented.
A declaration of conformity for medical devices is a formal document that certifies a device meets specific regulatory requirements. This statement assures stakeholders that the product complies with applicable standards, including safety and performance. In the context of the GA disclosure statement for medical device security, this declaration plays a vital role in demonstrating compliance with security protocols.
Although the MDS2 was not required by law, it was recommended by various regulatory bodies and industry organizations such as the FDA, the International Electrotechnical Commission (IEC), and the Association for the Advancement of Medical Instrumentation (AAMI).
The Manufacturer Disclosure Statement for Medical Device Security, generally abbreviated MDS2 (or MDS²), gives healthcare providers important cybersecurity information so they can evaluate the security capabilities of their devices or compare new devices when making product selections.
Consists of the MDS2 form and instructions for completing it. Assists professionals responsible for security-risk assessment in the management of medical device security issues.
The form is manufacturer-completed and provided to healthcare delivery organizations upon request. It's the buyer's responsibility to request an MDS2. It is not up to the manufacturer to provide it. The MDS2 form is entirely optional.