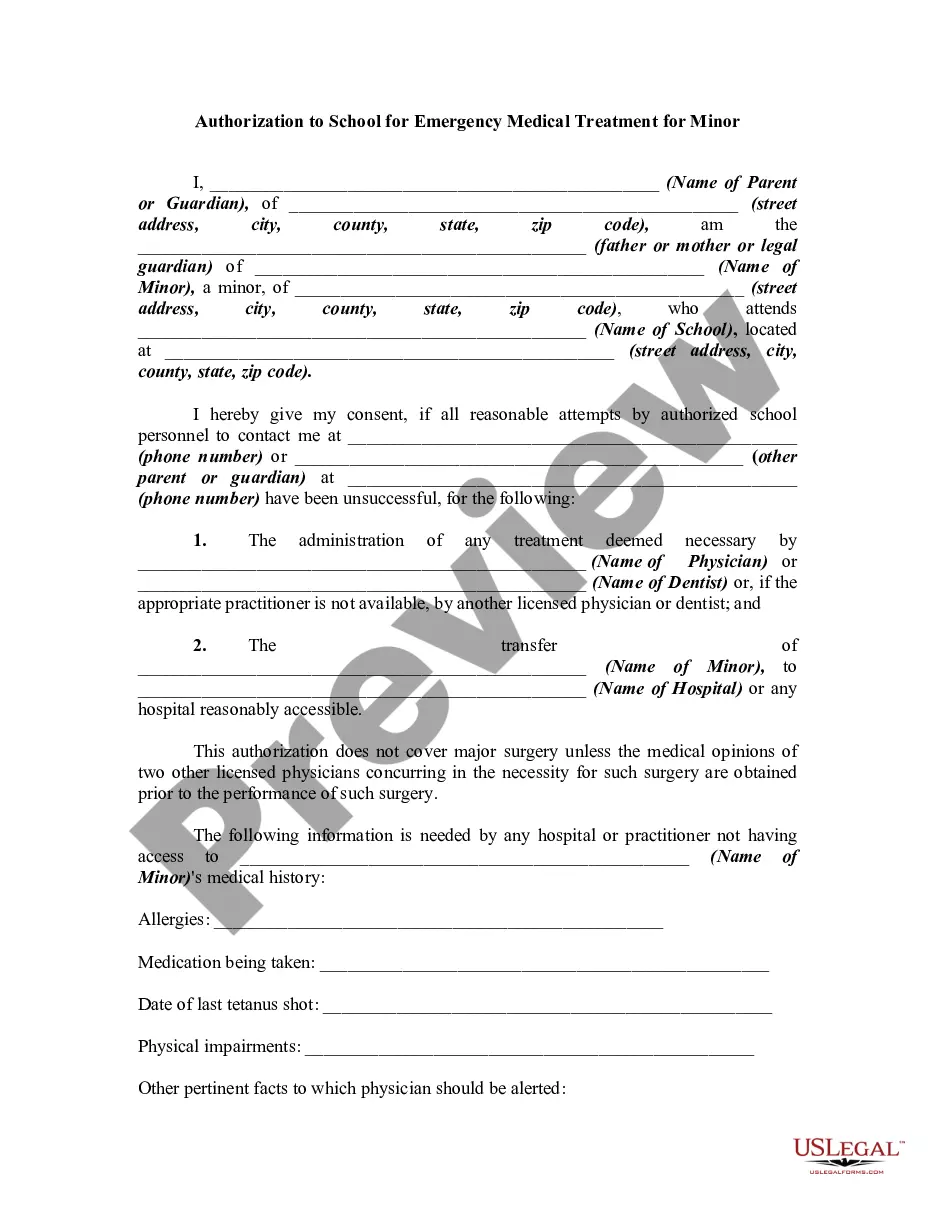
Authorization Medical Consent Without
Description
How to fill out Authorization To School For Emergency Medical Treatment For Minor - Patient Consent?
It’s clear that you can’t turn into a legal specialist instantly, nor can you understand how to swiftly draft Authorization Medical Consent Without without possessing a specialized background.
Assembling legal documents is a lengthy procedure that demands specific training and expertise. So why not entrust the preparation of the Authorization Medical Consent Without to the experts.
With US Legal Forms, one of the most comprehensive legal template collections, you can find anything from court documents to templates for internal corporate communication.
Restart your search if you need a different template.
Create a free account and choose a subscription plan to buy the template.
- We recognize how crucial compliance and adherence to federal and state regulations are.
- That’s why, on our site, all forms are localized and current.
- Here’s how you can begin with our website and acquire the form you need in just minutes.
- Locate the form you require by utilizing the search bar at the top of the page.
- Preview it (if this option is available) and review the supporting description to determine if Authorization Medical Consent Without is what you’re looking for.
Form popularity
FAQ
I have been explained; I have been provided with the requisite information; I have understood; and thereafter I consent, authorize and direct the above named doctor-in-charge / principal surgeon / principal interventionist and his / her team with associates or assistants of his / her choice to perform the proposed ...
Consent must be taken from the patient himself The doctor before performing any procedure must obtain patient's consent.
Consent refers to the patient's giving permission for electronic medical records to be released to third parties involved in treatment, utilization review, insurance payment, quality assurance, and continuity of care. Authorization is required for all other uses to which a patient's medical records may be put. What Is Consent? What Is Authorization? - Psychiatric News Psychiatric News ? doi ? full Psychiatric News ? doi ? full
A: ?Consent? is a general term under the Privacy Rule, but ?authorization? has much more specific requirements. The Privacy Rule permits, but does not require, a CE to obtain patient ?consent? for uses and disclosures of PHI for treatment, payment, and healthcare operations.
The consent form must include: A statement that the study involves research. ... Purpose of the research. ... Procedures. ... Risks or discomforts to the subject. ... Benefits of the research to the subject. ... Treatment Alternatives. ... Costs of Participation. ... Confidentiality.