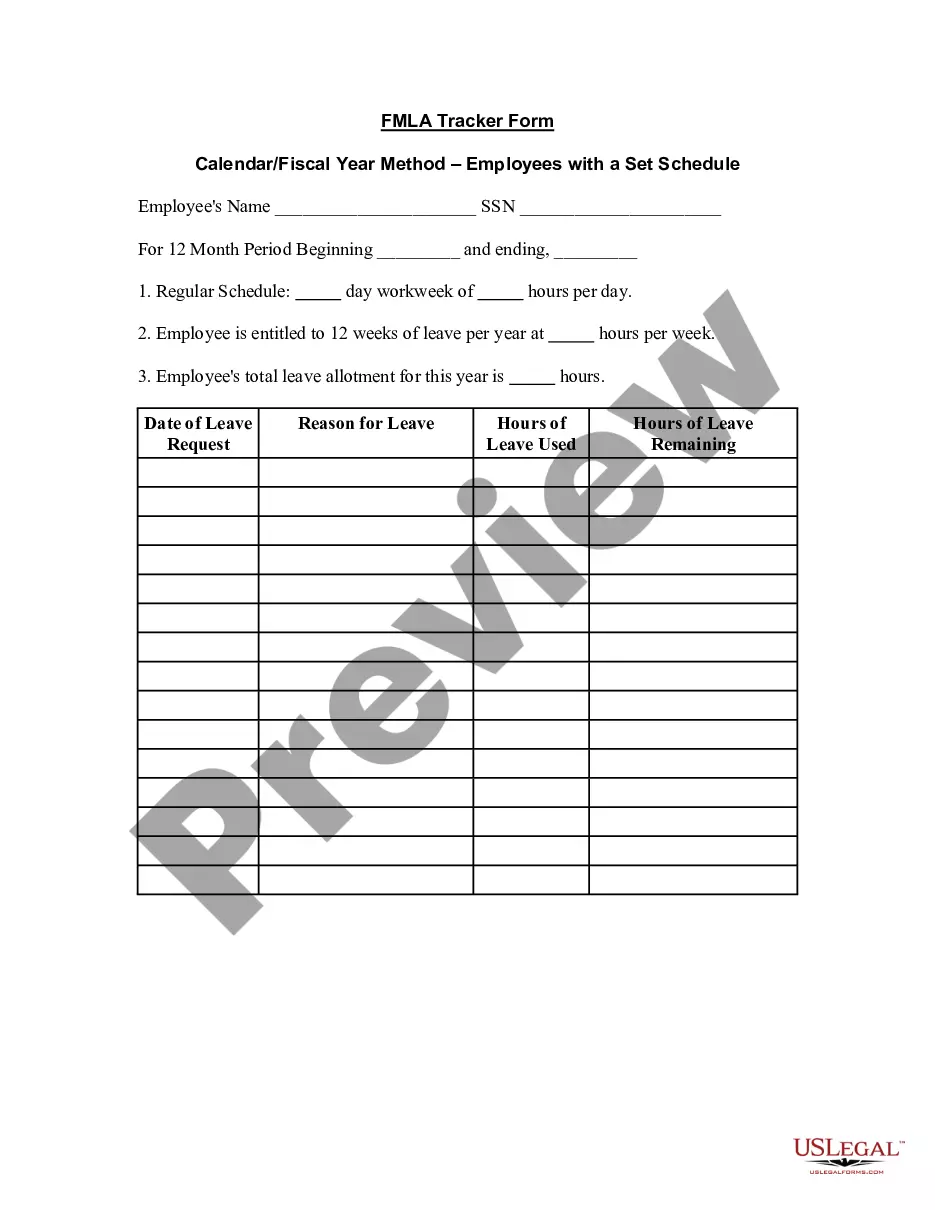
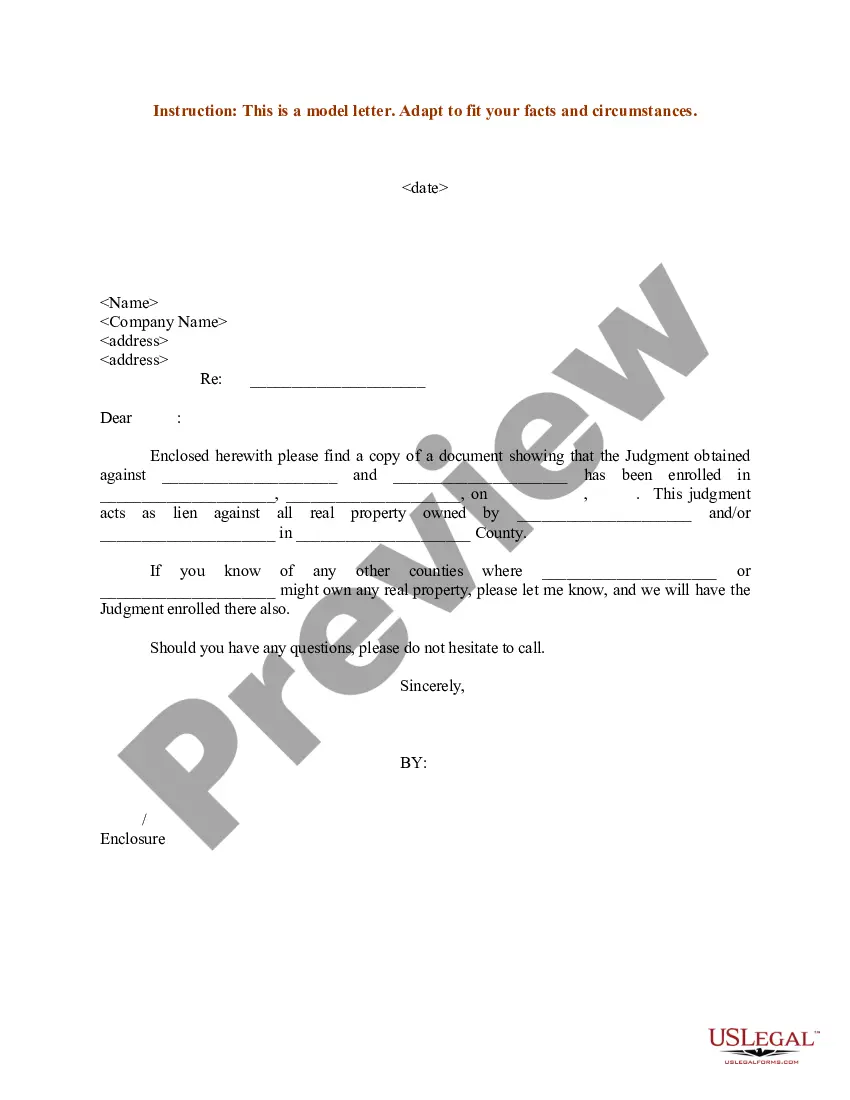
Consent Release Form Withdrawal In Phoenix
Description
Form popularity
FAQ
What is Arizona's age of consent? The age of consent in Arizona is 18 years old. This means that any person under the age of 18 is legally incapable of consenting to sexual conduct. Engaging in sexual activity with someone below this age can result in serious criminal charges, even if the minor willingly participated.
The consent form should include the following statements: I confirm that I have had the project explained to me, and have read the participant information sheet, which I may keep for my records. I have been given the opportunity to ask questions and have had them answered to my satisfaction.
Obtaining consent involves explaining the research and assessing participant comprehension using a consent document, usually a written consent form or information sheet, as a guide for the verbal explanation of the study.
(in-FORMD kun-SENT) A process in which patients are given important information, including possible risks and benefits, about a medical procedure or treatment, genetic testing, or a clinical trial. This is to help them decide if they want to be treated, tested, or take part in the trial.
Consent and release forms are given to your talent (interviewees, models, actors, etc.) and grants you permission to use their image (in video or photo form), audio, and their words in your production. Interview consent forms seek permission from the subject to use their image, audio, and dialogue.
The informed consent process involves three key features: (1) disclosing to potential research subjects information needed to make an informed decision; (2) facilitating the understanding of what has been disclosed; and (3) promoting the voluntariness of the decision about whether or not to participate in the research.
The patient must: o Be advised of diagnosis (if known) o Be advised of the general knowledge and purpose of the procedure o Be advised of the alternatives to such procedure; o Be advised of the associated risks and benefits of such procedure; o Have all questions answered regarding the procedure; and o Provide written ...
Consent is informed if the person giving the consent has been informed of and comprehends the nature, purpose, consequences, risks and benefits of the alternatives to the procedure, and has been informed and comprehends that withholding or withdrawing consent will not prejudice the future provision of care and services ...