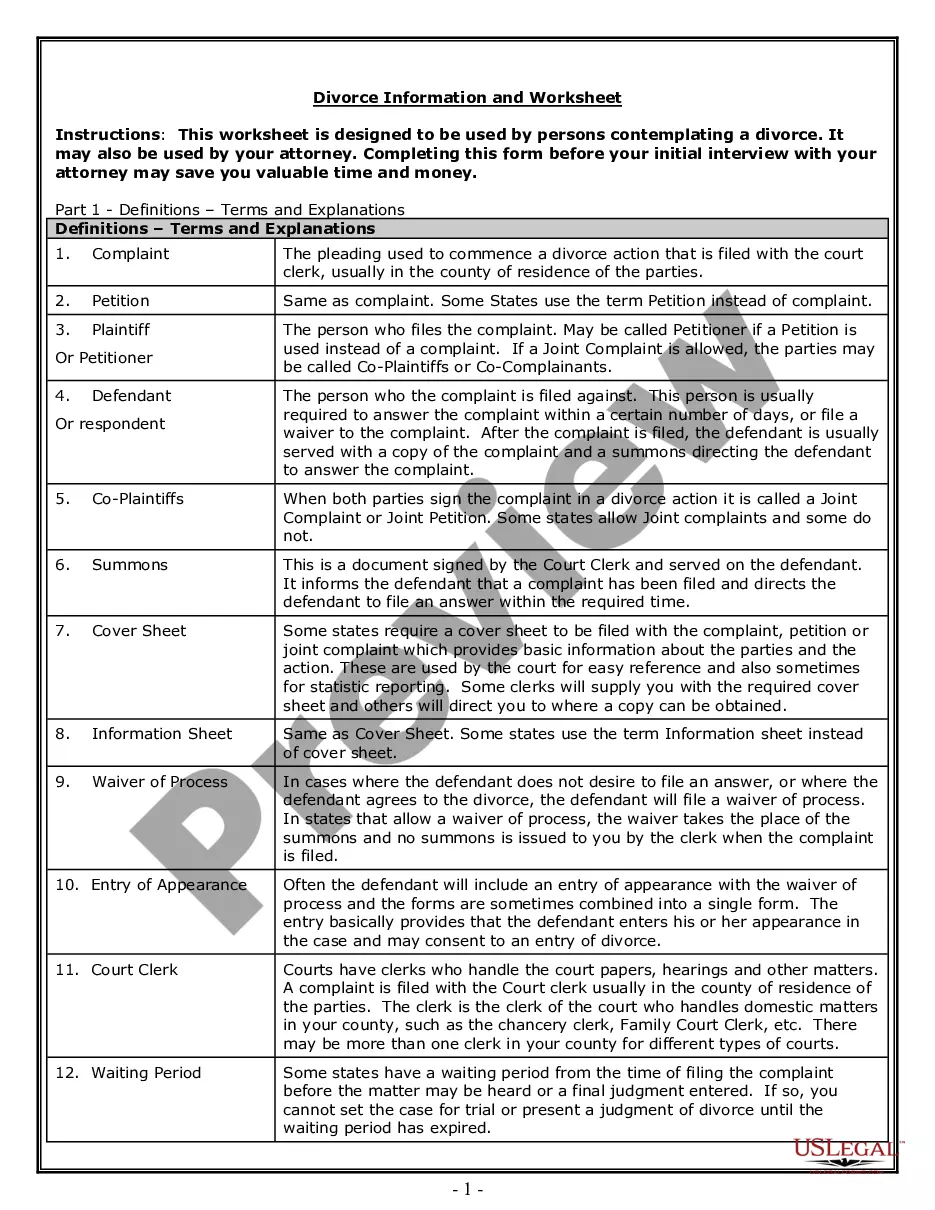
Release Of Medical Information Consent Form Template In Queens
Description
Form popularity
FAQ
A waiver is legal document releasing or relinquishing a known right, claim, or privilege. In this context, it is the relinquishment to pursue a claim in a certain set of defined circumstances. Informed consent is a written acknowledgement that a participant understands the risks inherent in a particular activity.
Forms are used to collect information from your customers, whereas Waivers and Policies are documents that your customers need to read and sign.
Obtaining informed consent in medicine is a process that should include describing the proposed intervention, emphasizing the patient's role in decision-making, discussing alternatives to the proposed intervention, discussing the risks and benefits of the proposed intervention, and eliciting the patient's preference, ...
With a waiver of documentation of consent, there is still a consent document and consent process, but subjects do not sign the document. Instead, subjects verbally agree to participate, or click a button in a web-based survey or complete the research activities.
Online Informed Consent: Best Practices Copy and paste the text of the IRB approved version of your informed consent document into Qualtrics. Create a link where participants can download the PDF version of the consent document at the time they are reading and/or electronically signing.
An Informed Consent is an alternative to a waiver. Parties signing this document are only consenting to the known and foreseeable physical risks inherent in the activity and not to the legal risks of negligence. Informed consents can be a good deterrent to legal action.
Waivers (also known as releases) are written agreements that say the sponsor of an activity will not be liable for harm suffered by participants. Although waivers are primarily legal tools, they also serve an educational purpose by making people think about the potential risks of an activity.
Instructions for Developing an Informed Consent Document General Information. Describe the purpose(s) of this research study in lay terms. Purpose of the Study. Procedures. Risks. Benefits. Compensation, Costs and Reimbursement. Withdrawal or Termination from Study. Confidentiality.
The informed consent document should succinctly describe the research as it has been presented in the IRB application. Use the second (you) or third person (he/she) to present the study details. Avoid use of the first person (I). Include a statement of agreement at the conclusion of the informed consent document.
Informed Consent Checklist (1998) A statement that the study involves research. An explanation of the purposes of the research. The expected duration of the subject's participation. A description of the procedures to be followed. Identification of any procedures which are experimental.