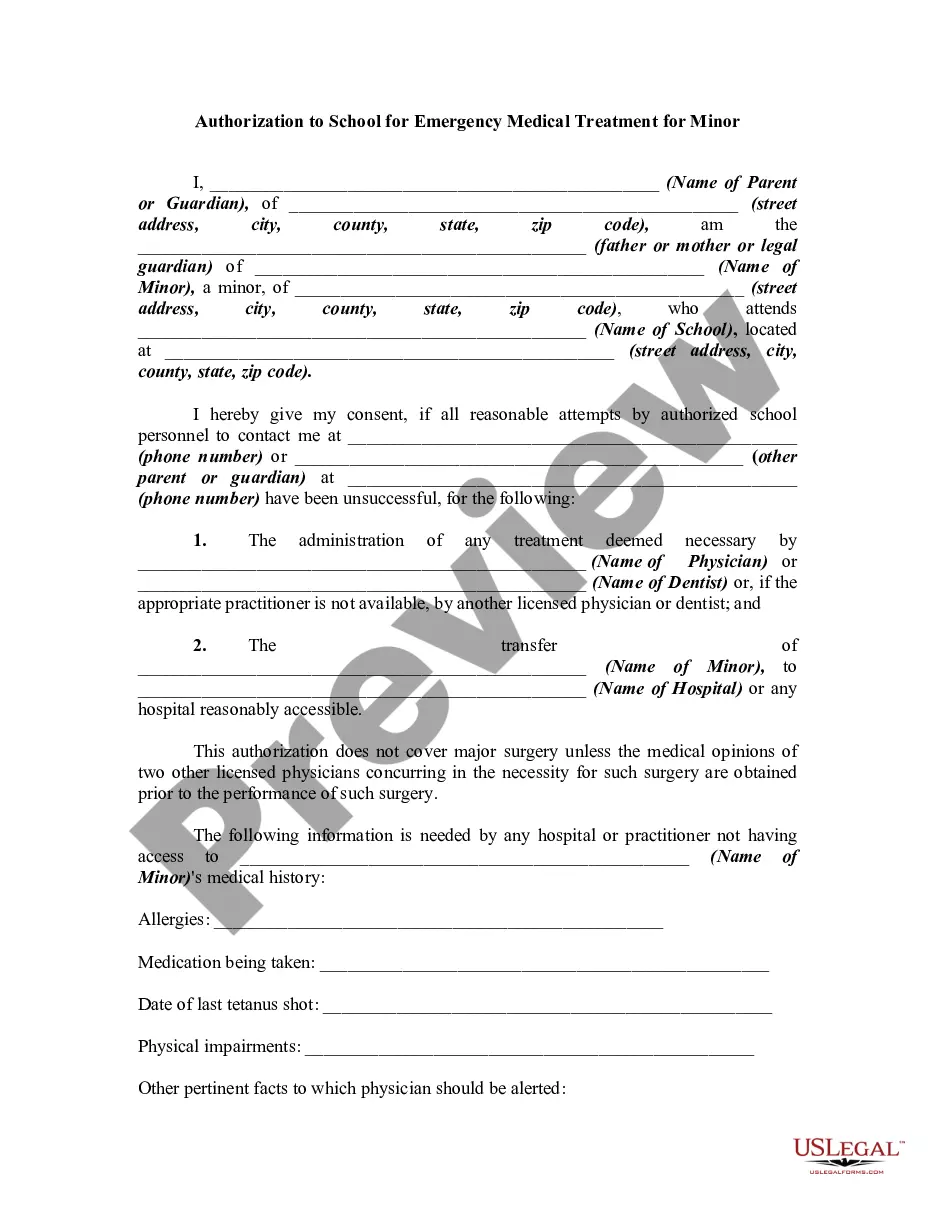
Consent Parent Document Format
Description
How to fill out Consent By Parent Or Guardian For Minor To Spend Weekend With Adult Not Related To Minor And Consent To Medical Care If Necessary?
Creating legal documents from the ground up can sometimes be intimidating.
Certain situations may require extensive investigation and significant expenditure.
If you're seeking a more straightforward and budget-friendly method of producing Consent Parent Document Format or any other documents without unnecessary hassle, US Legal Forms is always available to assist you.
Our online collection of over 85,000 current legal forms addresses nearly every aspect of your financial, legal, and personal affairs. With just a few clicks, you can promptly obtain state- and county-compliant forms meticulously prepared for you by our legal experts.
Examine the form preview and descriptions to ensure you are accessing the correct document. Ensure the template you select adheres to the rules and regulations of your state and county. Choose the most suitable subscription option to obtain the Consent Parent Document Format. Download the file, then complete, sign, and print it. US Legal Forms enjoys a solid reputation and over 25 years of experience. Join us today and transform document completion into a simple and efficient process!
- Utilize our platform whenever you require dependable and trustworthy services to swiftly locate and download the Consent Parent Document Format.
- If you are already familiar with our website and previously created an account with us, simply Log In to your account, find the template, and download it or re-download it at any time later in the My documents section.
- Not registered yet? No problem. Setting up an account takes only minutes to explore the selection.
- However, before proceeding to download the Consent Parent Document Format, be sure to follow these suggestions.
Form popularity
FAQ
When filling out parental consent, begin by gathering all relevant details, including the child's name, the parent's information, and the specific consent being granted. It is essential to write clearly and accurately in the Consent parent document format to avoid any misunderstandings. US Legal Forms offers templates that guide you through this process, ensuring that you include all required elements while maintaining legal compliance. This way, you can feel confident that your document is complete and effective.
To fill out a consent form example, start by identifying the specific information required, such as names, dates, and signatures. Make sure to include a clear statement of consent that outlines the purpose of the agreement. Use the Consent parent document format provided by US Legal Forms to ensure that you cover all necessary details and comply with legal standards. This approach not only simplifies the process but also enhances the validity of your document.
A simple example of consent could be a parent allowing their child to participate in a school field trip. The consent would include the child's name, details of the trip, and a statement indicating that the parent agrees to the child's participation. This example emphasizes the importance of using a clear consent parent document format to avoid confusion.
Writing a child consent form requires you to specify the child's details and the service or activity for which consent is sought. Clearly explain the implications of the consent, including any risks involved. Make sure the form is easy to understand and includes a section for the parent’s signature. A proper consent parent document format ensures all critical information is covered.
To create a parental consent form, start by defining the purpose and including necessary details such as the child's name and the activity. Use clear language to explain what the parents are consenting to, and provide space for them to sign. This form should be structured properly to ensure legality, and adopting a standard consent parent document format can simplify this task.
Writing a consent document involves outlining the purpose clearly, stating the parties involved, and specifying what is being consented to. Include relevant details like dates and conditions if applicable. Ensure that there is a space for the person's signature to confirm their understanding and agreement. Using an established consent parent document format can help streamline this process.
When filling out a parent consent letter, start with a formal greeting, then state the purpose of the letter. Clearly outline the details of the consent being given, including the child’s information and what activities or decisions it covers. End the letter with a space for the parent's signature and date. A well-organized consent parent document format enhances the letter's effectiveness.
To write consent from your parents, begin by outlining the specific reason for the consent. Include your full name, the nature of the consent, and any relevant dates. Make sure your parents sign the document to validate their agreement. Utilizing a clear consent parent document format can help prevent misunderstandings.
Filling out a parent consent form requires you to enter information such as the child's name, the activity or service being consented to, and the duration of the consent. Be sure to read all sections carefully and provide accurate details. After completing the form, have the parents sign and date it. Using a structured consent parent document format simplifies this process.
To write a consent form from parents, start by clearly stating the purpose of the consent. Include essential details like the child's name, date of birth, and specific activities covered by the consent. Finally, provide a section for parents to sign and date, ensuring they understand what they are consenting to. Using the right consent parent document format will help ensure clarity and legality.