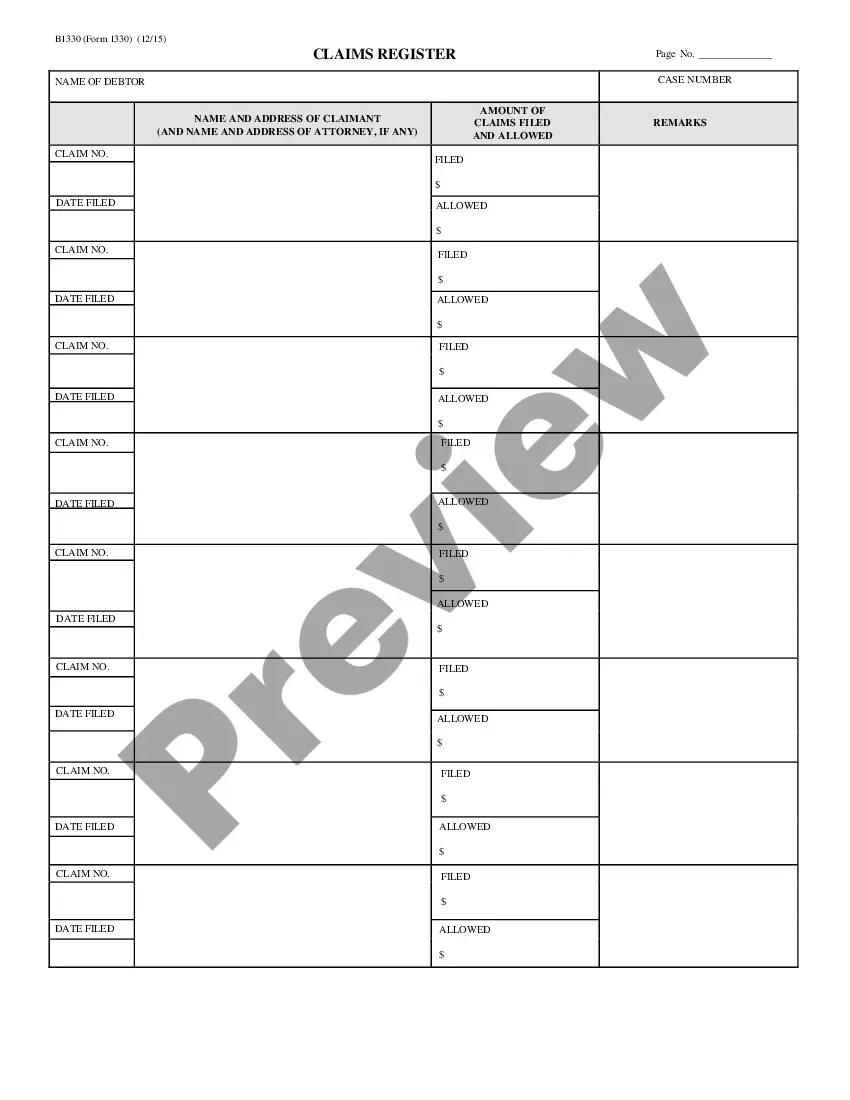
Consent Release Form Withdrawal In Wayne
Description
Form popularity
FAQ
Follow these steps to write an effective consent form. Step 1: Title and introduction. Step 2: Description of the activity. Step 3: Risks and benefits. Step 4: Confidentiality and data handling. Step 5: Voluntary participation and withdrawal. Step 6: Consent statement. Step 7: Signature and date. Step 8: Contact information.
Basic Elements of Informed Consent Purpose of the Research. Description of the Research. Risks. Benefits. Alternatives to Participation.
I participant name, agree to participate or agree to participation of my child participant name in the research project titled project title, conducted by researcher(s) name who has (have) discussed the research project with me. I have received, read and kept a copy of the information letter/plain language statement.
A document with important information about a medical procedure or treatment, a clinical trial, or genetic testing. It also includes information on possible risks and benefits. If a person chooses to take part in the treatment, procedure, trial, or testing, he or she signs the form to give official consent.
Examples of giving nonverbal consent may include: Head nod. Thumbs up. Pulling someone closer. Nodding yes. Making direct eye contact. Actively touching someone. Initiating sexual activity.
It must be obvious that the individual has consented, and what they have consented to. This requires more than just a confirmation that they have read terms and conditions – there must be a clear signal that they agree. If there is any room for doubt, it is not valid consent.
Instructions for Developing an Informed Consent Document General Information. Describe the purpose(s) of this research study in lay terms. Purpose of the Study. Procedures. Risks. Benefits. Compensation, Costs and Reimbursement. Withdrawal or Termination from Study. Confidentiality.
I participant name, agree to participate or agree to participation of my child participant name in the research project titled project title, conducted by researcher(s) name who has (have) discussed the research project with me. I have received, read and kept a copy of the information letter/plain language statement.
By signing a consent form, the client acknowledges their understanding of the treatment and the risks involved and gives their informed consent for the service to proceed. Release forms are legal documents that limit the spa from liability in case of unforeseen consequences that may arise during or after the treatment.
What is a Release Form? A release form, or general release form, is a legal document that serves as consent in writing to release the legal liability of a releasee by a releasor. The document is a formal acknowledgment that, once signed, is a legal release of all a releasee obligations within an agreement.