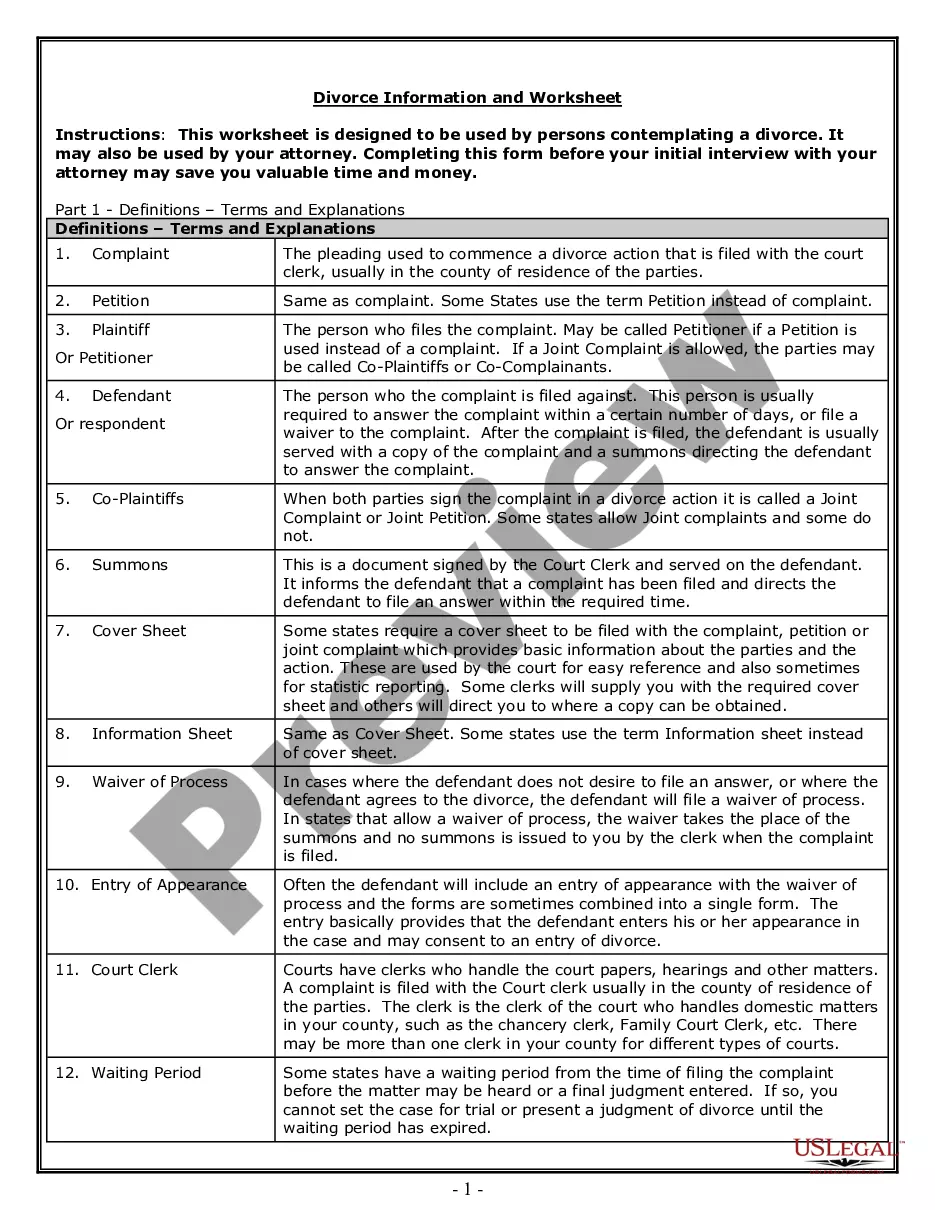
Employee Consent Form Sample In Philadelphia
Description
Form popularity
FAQ
How to write a consent form: A step-by-step guide Step 1: Title and introduction. Step 2: Description of the activity. Step 3: Risks and benefits. Step 4: Confidentiality and data handling. Step 5: Voluntary participation and withdrawal. Step 6: Consent statement. Step 7: Signature and date. Step 8: Contact information.
The five key elements of consent are: the individual gives consent voluntarily. the individual is adequately informed before giving consent. the consent is specific. the consent is current. the individual has the capacity to understand and communicate their consent.
A consent letter should include the title, sender and recipient's details, date, statement of consent, relevant details or conditions, acknowledgment of risks (if applicable), and signature.
How to write a consent form: A step-by-step guide Step 1: Title and introduction. Step 2: Description of the activity. Step 3: Risks and benefits. Step 4: Confidentiality and data handling. Step 5: Voluntary participation and withdrawal. Step 6: Consent statement. Step 7: Signature and date. Step 8: Contact information.
I participant name, agree to participate or agree to participation of my child participant name in the research project titled project title, conducted by researcher(s) name who has (have) discussed the research project with me. I have received, read and kept a copy of the information letter/plain language statement.
Informed consent language should be written in the second person (“you”), not in the first person (“I”). Minimize passive voice to the extent possible. Example of passive voice: “A summary of results will be sent to all study participants.” Example of active voice: “We will send you a summary of the results.”
Instructions for Developing an Informed Consent Document General Information. Describe the purpose(s) of this research study in lay terms. Purpose of the Study. Procedures. Risks. Benefits. Compensation, Costs and Reimbursement. Withdrawal or Termination from Study. Confidentiality.
How to fill out how to fill consent? Begin by identifying the parties involved in the consent form. Describe the purpose of the consent. Specify any limitations or restrictions associated with the consent. Make sure to clearly state who is giving consent and their capacity to do so.
I consent to participate in the research project and the following has been explained to me: the research may not be of direct benefit to me. my participation is completely voluntary. my right to withdraw from the study at any time without any implications to me.
I consent to participate in the research project and the following has been explained to me: the research may not be of direct benefit to me. my participation is completely voluntary. my right to withdraw from the study at any time without any implications to me.