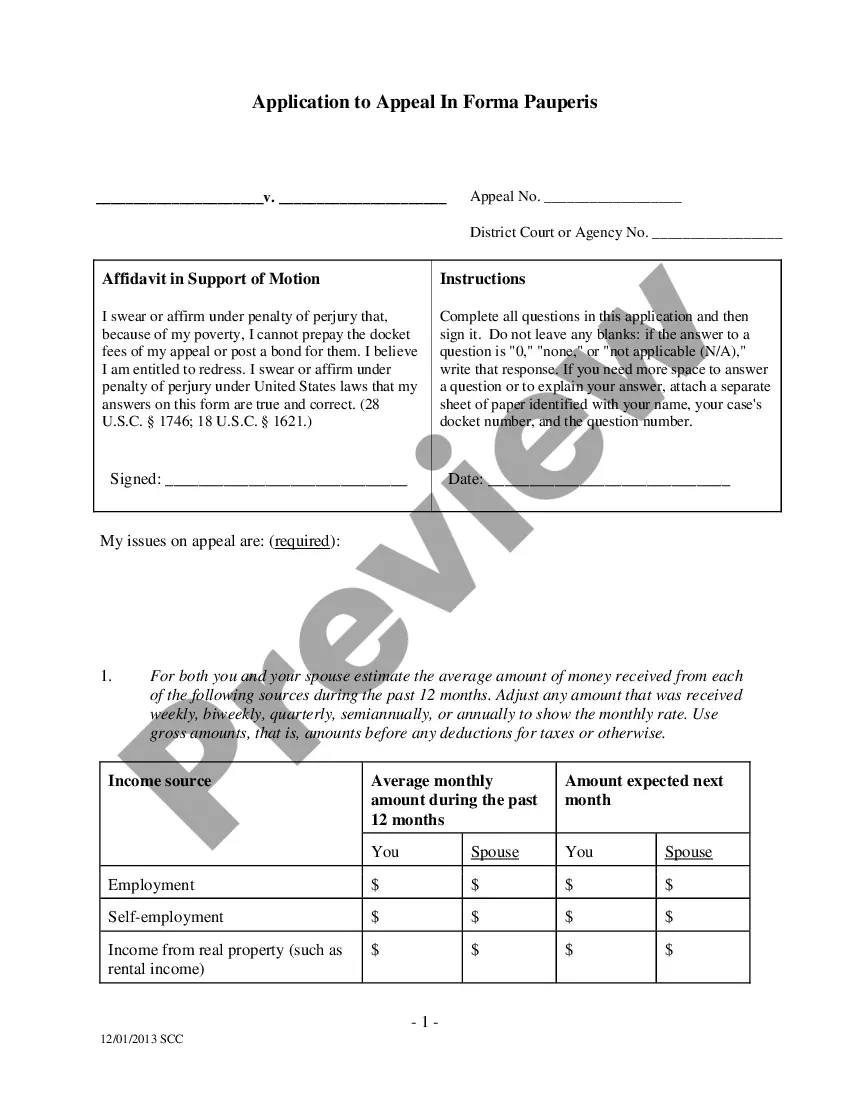
Employee Consent Form Meaning In Chicago
Description
Form popularity
FAQ
Participants would still need to be presented with the consent information, but would be informed that their consent is implied by submitting the completed survey. For confidential online surveys, researchers may place the consent in the survey and request the subject select an "I agree" or "I do not agree" checkbox.
Written consent If you're using an online form or document instead of printed copies, you can email participants a copy. It can be helpful to send the consent form to people in advance, so that they have the time to read and understand the information.
Defining consent informed – the person must be given all of the information about what the treatment involves, including the benefits and risks, whether there are reasonable alternative treatments, and what will happen if treatment does not go ahead.
The Consent Form provides an employer's disclosure of information, rights and rules pertaining to the background check and obtains the consumer's authorization to run the background check.
Online Informed Consent: Best Practices Copy and paste the text of the IRB approved version of your informed consent document into Qualtrics. Create a link where participants can download the PDF version of the consent document at the time they are reading and/or electronically signing.
There are various types of consent, including explicit consent, implied consent, opt-in consent, and opt-out consent.
As technology has advanced, electronic (and digital) signature has become acceptable for documenting legally effective informed consent if implemented correctly by the study team. The Board can grant a waiver of documentation of consent for studies which have been determined to be no more than minimal risk.
Consent should be obtained before the participant enters the research (prospectively), and there must be no undue influence on participants to consent. The minimum requirements for consent to be informed are that the participant understands what the research is and what they are consenting to.
I participant name, agree to participate or agree to participation of my child participant name in the research project titled project title, conducted by researcher(s) name who has (have) discussed the research project with me. I have received, read and kept a copy of the information letter/plain language statement.
All sections of the consent form, except the "Consent" section, should be written in second person ("You are invited..."). Headers should include “Informed Consent” followed by the title of the study (e.g., the header in this document). Footers should include page numbers.