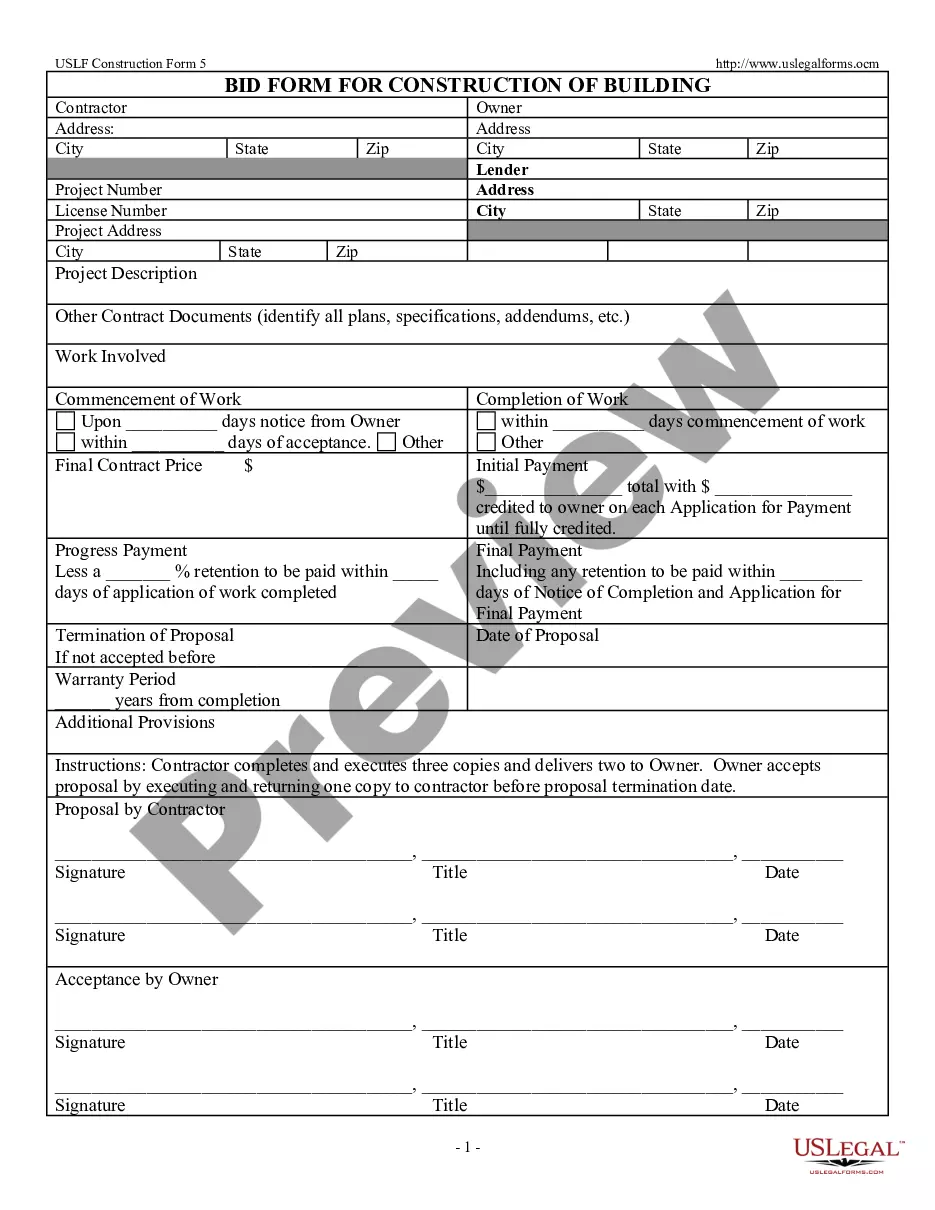
Offer To Sell Sample Formula In Tarrant
Description
Form popularity
FAQ
The requirement that all listing agreements have a definite expiration date is typically the responsibility of state real estate licensing laws and regulations. Each state has its own laws and regulations governing real estate transactions, including listing agreements between sellers and real estate agents.
A listing agreement is between the parties that own a property and the agents or brokers who will find a buyer for it. Typically, a real estate listing agreement involves the property owner and a real estate agent. The property owner, or seller, grants the agent the right to market and sell the property.
"Exclusive right to sell" is a type of listing contract you enter into with a real estate agent. Put simply, it says that the signing agent is the only person allowed to market and sell your property for a certain amount of time. Generally, these agreements last anywhere from one to six months.
A listing agreement is between the parties that own a property and the agents or brokers who will find a buyer for it. Typically, a real estate listing agreement involves the property owner and a real estate agent. The property owner, or seller, grants the agent the right to market and sell the property.
For most parents, throwing away these unopened and unexpired bottles is the normal course of action. Sell-Formula aims to buy back these extra baby formulas and make sure that they don't go to waste.
The deal over here is, you have no business selling baby formula ANYWHERE if you are not a retailer buying directly from a brand or brand authorized wholesale distributor. (AKA you have a legit business!)
There are FB groups for selling and trading formula. You can't sell it on marketplace, but I'd try the formula groups. You can also ask in local mom groups if anybody wants to sell or trade for the kind your baby does tolerate.
In addition, as discussed more fully below, section 412 of the Act requires persons responsible for the manufacture or distribution of infant formula to register with FDA and to make a submission to FDA for any new infant formula, which includes any infant formula that has had a major change in its formulation or ...