Order Setting Bond For F2
Description
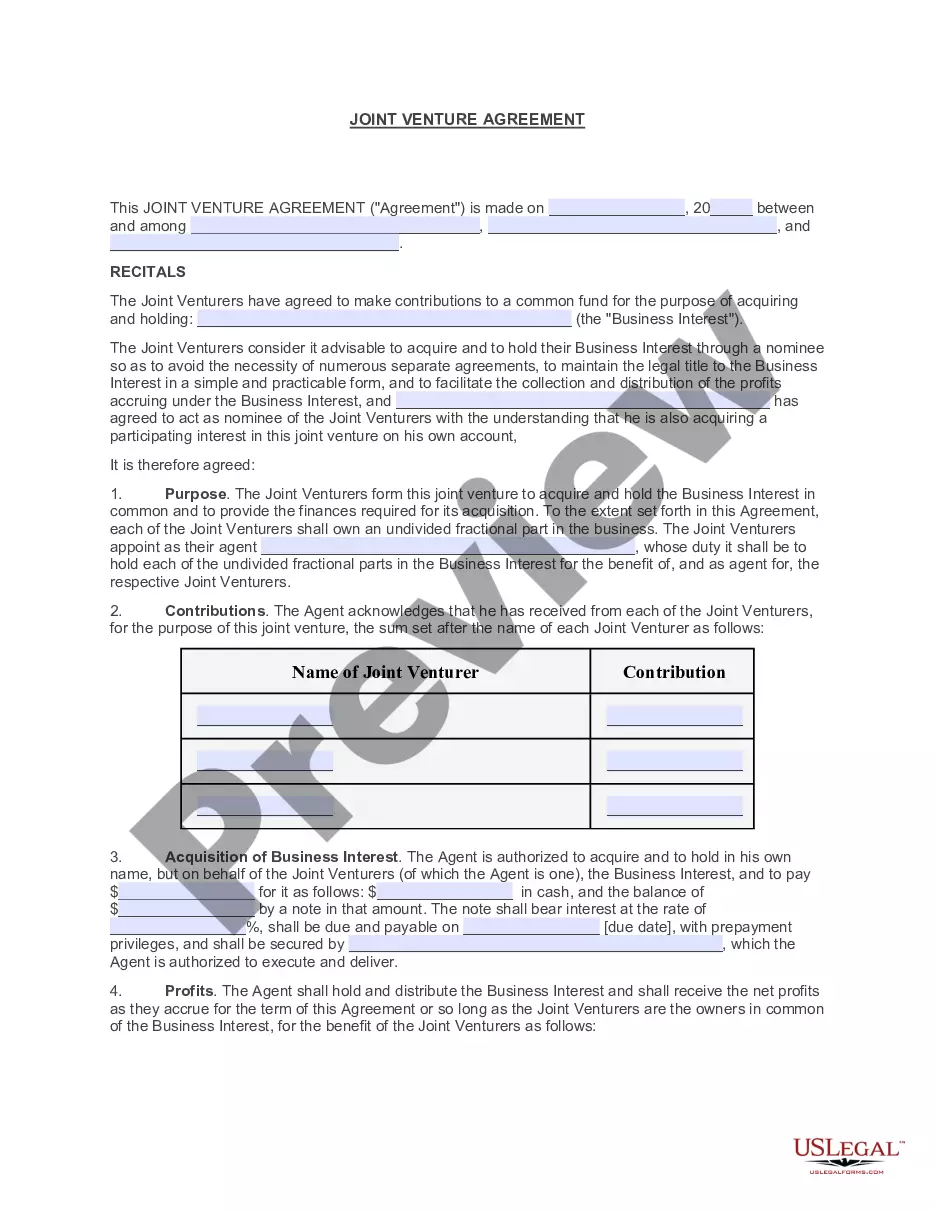
How to fill out Order Setting Bond?
Managing legal documents and processes can be a lengthy addition to your entire day.
Order Setting Bond For F2 and similar forms typically necessitate you to search for them and comprehend how to fill them out accurately.
Consequently, if you are addressing financial, legal, or personal issues, possessing a comprehensive and accessible online repository of forms readily available will greatly assist.
US Legal Forms is the premier online platform for legal templates, providing over 85,000 state-specific forms and a variety of resources to help you complete your documents swiftly.
Is this your first time using US Legal Forms? Sign up and create your account in a few moments, and you’ll gain access to the form library and Order Setting Bond For F2. Then, follow the steps below to complete your form: Ensure you have located the correct form by using the Review feature and examining the form description. Select Buy Now when you are ready, and choose the monthly subscription plan that fits your needs. Click on Download then fill out, sign, and print the form. US Legal Forms has 25 years of experience helping users manage their legal documents. Find the form you need right now and streamline any process effortlessly.
- Explore the collection of pertinent documents accessible to you with just a single click.
- US Legal Forms provides you with state- and county-specific forms available for download at any time.
- Protect your document management tasks using a premium service that enables you to prepare any form within minutes without incurring extra or hidden fees.
- Simply Log In to your account, find Order Setting Bond For F2, and download it immediately from the My documents section.
- You can also access previously downloaded forms.
Form popularity
FAQ
F2 and O22? have bond order 1 while N2,CO and NO+ have bond order 3.
Fluorine molecule,F2, has 18 electrons in total..it has 10 bonding electrons and 8 anti bonding electrons .. therefore its bonding order is 1.
The bond order is 2 (double bond). In ethylene H 2C=CH 2 the bond order between the two carbon atoms is also 2. The bond order between carbon and oxygen in carbon dioxide O=C=O is also 2. In phosgene O=CCl 2, the bond order between carbon and oxygen is 2, and between carbon and chlorine is 1.
Bond order (B.O.) is defined as one-half the difference between the number of electrons present in the bonding and the anti-bonding orbitals. Bond order (B.O) = ( Number of bonding electrons - Number of the anti-bonding electron) Bond order = (Nb- Na)
Summary: ing to the molecular orbital theory, the F2 molecule consists of one sigma bond (?1s) and two pi bonds (?2px and ?2py). The sigma bond is formed by the head-on overlap of the 1s orbitals, while the pi bonds are formed by the sideways overlap of the 2p orbitals.