Loading
Get Ny Wadsworth Center Certificate Of Qualification Questionnaire Virology 2020-2025
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the NY Wadsworth Center Certificate Of Qualification Questionnaire Virology online
Filling out the NY Wadsworth Center Certificate Of Qualification Questionnaire Virology online is an important step for individuals involved in virology testing. This guide provides clear instructions to help you navigate each section of the form effectively.
Follow the steps to complete the questionnaire accurately.
- Click ‘Get Form’ button to access the questionnaire and open it in your online editor.
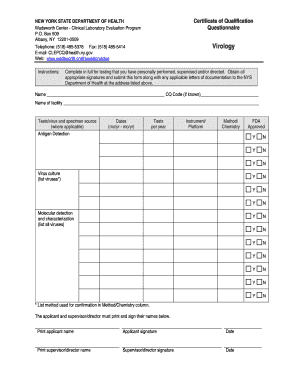
- Begin by entering your name in the designated field. If you have a CQ code, include it as well.
- Fill in the name of your facility in the specified section, ensuring accuracy for proper identification.
- In the 'Tests/virus and specimen source' section, indicate the types of tests you perform, including antigen detection, virus culture, and molecular detection. Fill in the applicable fields and list the specific viruses you work with.
- For each type of test mentioned, record the dates you conducted them in the format (mo/yr - mo/yr). This ensures a complete timeline of your testing history.
- Next, enter the number of tests you perform per year for each listed virus and specify the instruments or platforms you utilize for these tests.
- In the 'Method/Chemistry' section, describe the method used for confirmation for each test, clearly linking it to the appropriate virus.
- Indicate whether each method is FDA approved by marking 'Y' for yes or 'N' for no as appropriate.
- Once all sections are completed, proceed to the signature area. Both the applicant and supervisor/director must print and sign their names, followed by the date.
- After ensuring all information is accurate and complete, save the changes made to your form. You can then download, print, or share the form as needed.
Complete your documents online today for a streamlined process.
§ 493.1405 Standard; Laboratory director qualifications. The laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate complexity tests and must be eligible to be an operator of a laboratory within the requirements of subpart R of this part.
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


