Get Fda 481(e)-cg
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the FDA 481(E)-CG online
The FDA 481(E)-CG is an essential document used for compliance and inspection reporting in the food and drug industry. This guide provides clear, step-by-step instructions for filling out the form online, ensuring that users can navigate the process efficiently and accurately.
Follow the steps to successfully complete the FDA 481(E)-CG online.
- Click ‘Get Form’ button to obtain the form and open it in the editor.
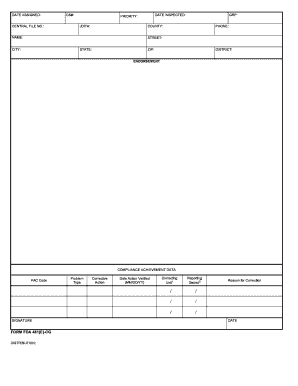
- Fill out the 'Date Assigned,' 'Central File No.,' and 'CS#' sections at the top of the form. Ensure that these fields are accurately completed to reflect the current status of the inspection.
- Enter the 'Date Inspected' and 'Priority' fields, which indicate when the inspection took place and its level of urgency.
- Proceed to the 'Name,' 'Phone,' 'Street,' 'City,' 'State,' and 'Zip' sections to provide the contact information for the establishment involved.
- Complete the 'District' section to specify the relevant district for the inspection.
- In the 'Compliance Achievement Data' section, fill out the 'PAC Code,' 'Problem Type,' and 'Corrective Action.' Provide the necessary details to reflect the compliance issues observed.
- Document the 'Date Action Verified' and 'Correcting Unit' to track when the corrective actions were implemented.
- Fill in the 'Reporting District' to indicate where the reports will be sent or processed.
- If applicable, add any 'Related Firms' information in the designated section.
- Address any 'Establishment Changes' and fill in the 'PAC' and 'Process Code' fields as necessary.
- Complete the 'Samples Collected' section, noting the 'Sample #' and 'Product' involved.
- Add inspector information by signing in the specified areas and including the names and signatures of both the inspector and supervisor.
- Review all entries for accuracy and completeness before moving to save changes, download, print, or share the form.
Complete your FDA 481(E)-CG form online today to ensure timely compliance and reporting.
FDA 483 letters are available through the FDA’s official website and other regulatory databases. You may also find them in the FDA’s compliance history documentation, which is accessible to the public. By searching with the relevant keywords, such as FDA 481(E)-CG, you can locate specific letters that pertain to your industry. This knowledge can assist in better compliance practices.
Industry-leading security and compliance
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


