Loading
Get 6.4 Electronic Structure Of Atoms (electron Configurations ...
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the 6.4 Electronic Structure Of Atoms (Electron Configurations) online
Filling out the 6.4 Electronic Structure Of Atoms (Electron Configurations) form online can be a straightforward process with the proper guidance. This document will provide a step-by-step approach to help you navigate each section of the form effectively.
Follow the steps to complete your electronic structure form.
- Click ‘Get Form’ button to obtain the form and open it in the editor.
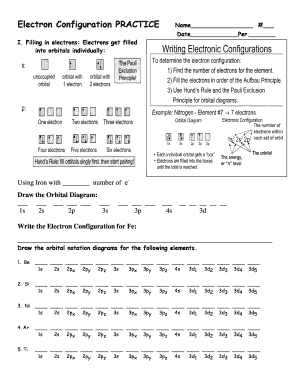
- Begin by filling in the personal information section at the top of the form, including your name, date, and period. This information helps to organize responses accurately.
- In the section regarding filling in electrons, you will need to understand the concept of orbitals. Use the provided spaces to input the orbital diagram for Iron (Fe) based on its electron configuration.
- Next, write the Electron Configuration for Iron. This involves specifying the electron distribution among the various orbitals.
- Proceed to draw the orbital notation diagrams for the specified elements such as Be, Si, Ni, Ar, and Ti. Follow the format given for placing electrons in orbitals properly.
- For the next part of the form, you will write the electron configurations for elements like Si, Cr, and Mg. Capture the complete distribution of electrons across the orbitals.
- Additionally, write the noble gas configuration for the specified elements. This is a more condensed form of the electron configuration, using the nearest noble gas as a reference.
- In the section for ions, draw the orbital diagrams while noting any added or removed electrons due to ionization. Use the example provided for Sodium as a reference.
- Complete the electron configuration for ions such as Be2+, Li1+, and F1- in the designated spaces.
- Finally, for the Valence Electrons section, draw the electron dots for Fe based on its electron configuration. Ensure to follow the specified instructions closely.
- Once you have filled out all sections, review your work for accuracy. Save your changes, and you may download, print, or share the completed form as needed.
Start filling out the 6.4 Electronic Structure Of Atoms (Electron Configurations) form online today for a better understanding of electron configurations.
Related links form
ElementAtomic numberElectron configuration carbon 6 1s22s22p2 nitrogen 7 1s22s22p3 oxygen 8 1s22s22p4 fluorine 9 1s22s22p514 more rows
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


