Loading
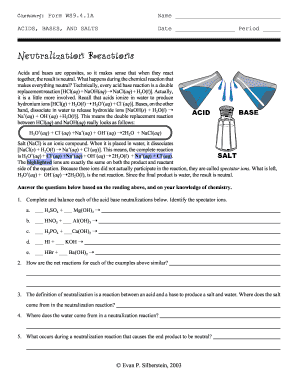
Get Neutralization Reactions Form Ws9 4 1a
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the Neutralization Reactions Form Ws9 4 1a online
This guide offers a clear, step-by-step approach to completing the Neutralization Reactions Form Ws9 4 1a online. Whether you are familiar with chemical concepts or new to the subject, this guide will assist you in accurately filling out the form.
Follow the steps to complete the form effectively.
- Click the ‘Get Form’ button to obtain the Neutralization Reactions Form Ws9 4 1a and open it in your online editing tool.
- Fill in your name in the designated space at the top of the form to identify yourself as the user.
- Enter the date in the provided field to record when the form is completed.
- Indicate your period in the space provided, which helps to categorize the form for your class or session.
- Read the section that explains neutralization reactions carefully. This background will be useful in answering the questions that follow.
- Proceed to the questions section. Complete and balance each acid-base neutralization reaction where indicated, and identify the spectator ions for each equation.
- Answer the following questions based on your understanding of neutralization reactions: similarities in net reactions, the origin of salt and water in reactions, and what makes the end product neutral.
- Once you have completed the form, review your answers for accuracy.
- Finally, save your changes, and choose to download, print, or share the completed form as needed.
Complete your document online to ensure all necessary information is accurately captured.
A neutralization reaction is when an acid and a base react to form water and a salt.
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


