Get Section D Cdc Continuation Page For Application For Permit To Import Biological Agents Or Vectors
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the Section D CDC continuation page for application for permit to import biological agents or vectors online
This guide provides comprehensive instructions for completing the Section D CDC continuation page, a crucial component of the application process for importing biological agents or vectors into the United States. By following these steps, users will ensure accurate and effective submission of their information.
Follow the steps to complete the form accurately and efficiently.
- Click the ‘Get Form’ button to obtain the form and open it in the editor.
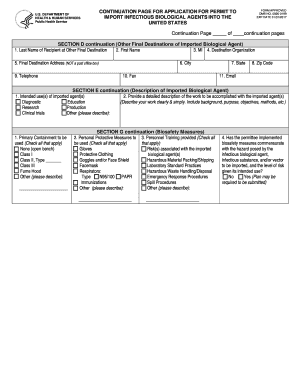
- Begin by filling out Section D, which pertains to other final destinations of the imported biological agent. Provide the last name, first name, and middle initial of the recipient at the destination organization.
- Input the destination organization's name in the specified field and provide a complete address, making sure to include the city, state, zip code, and any relevant contact numbers such as telephone and fax.
- Ensure you also include an email address for correspondence related to the import process.
- Proceed to Section E, which requires a description of the imported biological agent. Clearly state the intended uses of the agents, detailing the work to be accomplished, which may include diagnostic, education, research, production, clinical trials, or other uses.
- In this section, check all applicable boxes indicating the primary containment measures to be used during the handling of the biological agents.
- Next, navigate to Section G, which outlines biosafety measures. Check all protective measures that will be employed, such as gloves, protective clothing, goggles, and respirators.
- Indicate whether personnel have received appropriate training by checking all applicable options and providing details on any specific immunizations if relevant.
- Finally, confirm whether the permittee has implemented biosafety measures that are appropriate for the risks associated with the biological agents being imported.
- Once all fields are complete, review your entries for accuracy before saving the document. You will have the option to download, print, or share the completed form.
Begin your application process today by completing your documents online.
The purpose of an import permit is to regulate and monitor the entry of specific goods into a country, thereby protecting public health and safety. Import permits help to prevent the introduction of harmful substances and ensure compliance with national laws. For biological agents or vectors, the Section D CDC Continuation Page for Application for Permit to Import Biological Agents or Vectors outlines the necessary steps to obtain this essential documentation.
Industry-leading security and compliance
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


