Get Mds2 Form Hn 1-2013 2020-2025
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the MDS2 Form HN 1-2013 online
The MDS2 Form HN 1-2013 is essential for documenting the security measures associated with medical devices. This guide provides clear, step-by-step instructions to help users accurately complete the form online, ensuring compliance and clarity.
Follow the steps to effectively complete the MDS2 Form HN 1-2013.
- Press the ‘Get Form’ button to access the MDS2 Form HN 1-2013 and open it for online editing.
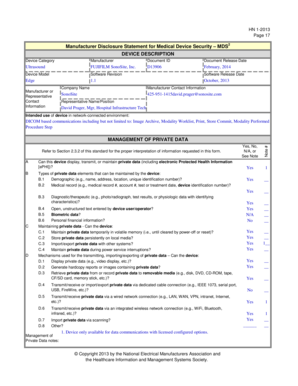
- Begin with the device description section. Fill in the device category (e.g., ‘Ultrasound’), manufacturer name, document ID, and the document release date. This section provides critical identification details.
- Complete the device model, software revision, and software release date. Enter ‘Edge’ for model, '1.1' for software revision, and the corresponding software release date.
- Provide the manufacturer or representative contact information. Include the company name, phone number, and email of the contact person for any inquiries.
- In the intended use section, describe how the device operates in a network-connected environment, specifically noting DICOM-based communications.
- Proceed to the section regarding private data. Answer yes or no to whether the device can display, transmit, or maintain private data, and specify the types of private data elements that the device can maintain.
- Detail the mechanisms used for transmitting and managing private data by filling out the respective fields. Ensure to address each specific question thoroughly.
- Review the sections related to security capabilities. Answer each question honestly based on the device capabilities such as auto-logoff features, audit controls, and authorization levels.
- Conclude by checking all sections for completeness. Save your changes and download the completed form. You may also choose to print or share it as needed.
Complete your MDS2 Form HN 1-2013 online today to ensure the security management of your medical devices.
The MDS2 form, or MDS2 Form HN 1-2013, stands for Manufacturer's Declaration of Conformity. This document outlines a manufacturer’s commitment to adhere to specific industry standards and regulations for medical devices. By submitting this form, manufacturers can facilitate smoother approval processes, making it easier to bring their products to market. Therefore, the MDS2 form is a vital tool for compliance in the medical device sector.
Industry-leading security and compliance
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


