Loading
Get Fda 2438 2003-2026
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the FDA 2438 online
Filling out the FDA 2438 form online is an important step for those involved in medical device compliance. This guide provides a clear, step-by-step process to help ensure that you correctly navigate and complete the form.
Follow the steps to successfully complete the FDA 2438 online.
- Click the ‘Get Form’ button to obtain the FDA 2438 form and open it in your browser.
- Begin by entering the basic information requested on the form, such as the manufacturer's name and address. Ensure the details are accurate to avoid any delays.
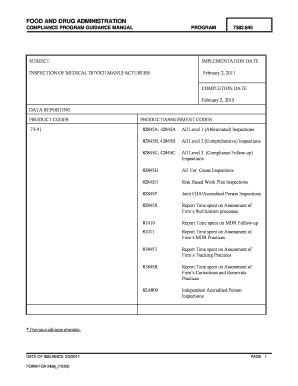
- Proceed to fill out the product codes and assignment codes specific to your inspection type. Refer to the table provided in the guidance for correct entries.
- In the sections regarding inspectional strategy, detail the applicable Quality System regulations being followed. This may involve summarizing processes and controls in place.
- For each reported outcome relevant to the medical devices, provide detailed descriptions. Highlight any adverse events, if applicable, as this is critical for regulatory compliance.
- After filling in all sections, review the form for completeness and accuracy. Correct any errors or omissions.
- Once satisfied that all entries are correct, save your changes. You can then download a copy, print the form, or share it as required.
Complete your FDA 2438 filing online today to ensure compliance with medical device regulations.
To submit a petition to the FDA, follow the guidelines provided for petitions on their website. Your petition must include a statement of your request, relevant data, and reasons supporting your position. For clarity and efficiency, refer to FDA 2438 to ensure you meet all requirements and enhance the likelihood of approval.
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


