Loading
Get Roche Srd-0120176
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the Roche SRD-0120176 online
Filling out the Roche SRD-0120176 form online is a crucial step for reporting adverse events and special situations. This guide will provide you with clear and detailed instructions to ensure accurate and effective completion of the form.
Follow the steps to successfully complete the Roche SRD-0120176 form.
- Click ‘Get Form’ button to obtain the Roche SRD-0120176 online and open it in your document editor.
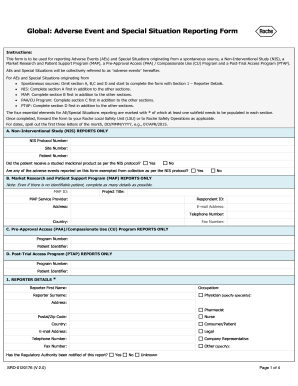
- Determine the type of report you are submitting. If it is from a spontaneous source, skip sections A, B, C, and D, and start by filling out Section 1 – Reporter Details.
- For Non-Interventional Study (NIS) reports, complete Section A first, in addition to the other sections. Ensure to input the NIS protocol number, site number, and patient number. Answer the questions regarding medicinal product receipt and adverse event exemptions accordingly.
- If you are reporting from a Market Research and Patient Support Program (MAP), fill out Section B first, including the MAP ID, project title, service provider, respondent ID, and contact details.
- For Pre-Approval Access (PAA) or Compassionate Use (CU) Program reports, begin with Section C, entering the program number and patient identifier.
- For Post-Trial Access Program (PTAP) reports, complete Section D first with the program number and patient identifier.
- Fill out Section 1 – Reporter Details, including your name, occupation, and contact information. It is essential that at least one subfield marked with * is populated.
- Continue to provide patient details in Section 3, including name, gender, date of birth, weight, height, and ethnic origin, ensuring accuracy.
- In Sections 4 and 5, report on the suspect products and adverse events, providing comprehensive details for each item listed.
- Document any concomitant medications in Section 6, along with test results in Section 7 if applicable.
- Utilize Section 8 for any additional relevant information, including descriptions of adverse events and treatment outcomes.
- Once all sections are completed, save changes, download, print, or share the form as needed by forwarding it to your Roche Local Safety Unit or Roche Safety Operations.
Start filling out the Roche SRD-0120176 online today to ensure timely reporting of adverse events.
When dealing with the Roche SRD-0120176, it is crucial to report any adverse events promptly. Generally, you have 15 days to report the event to the relevant authorities. Timely reporting is important for ensuring patient safety and effective monitoring of the medication. Using the uslegalforms platform can help streamline this process, making it easier to submit your reports.
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


