Loading
Get Certification Audit Observation Form
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the Certification Audit Observation Form online
This guide provides a clear and supportive overview of how to fill out the Certification Audit Observation Form online. Whether you are familiar with digital forms or new to the process, this step-by-step guide will help you navigate each section with ease.
Follow the steps to successfully complete the form.
- Click the ‘Get Form’ button to obtain the Certification Audit Observation Form. This action will open the form in an online editor for you to fill out.
- Begin by entering the application number at the top of the form. This number is essential for tracking and referencing your submission.
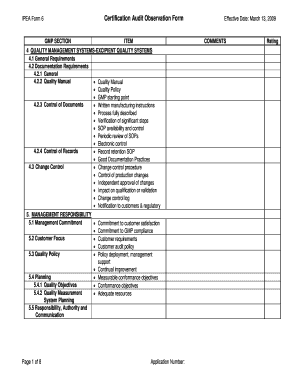
- Proceed to the GMP section, and carefully review each item listed under the applicable categories. Make sure to follow the prompts and provide the necessary comments or ratings.
- For each section, such as 'Quality Management Systems' or 'Management Responsibility,' fill in your observations, ensuring that you adhere to the guidelines for documentation and responses.
- In the comments section, you may provide additional insights or clarifications related to your observations for each item. Be specific and concise in your descriptions.
- Once you have completed all sections of the form, review your entries for accuracy and completeness. Check that you have addressed all relevant points.
- After ensuring that all information is correct, use the available options to save your changes, download a copy of the filled form, print it, or share it as needed.
Complete the Certification Audit Observation Form online today to ensure a thorough and accurate submission.
The form of the auditor's certificate is as follows: We, the undersigned, being the auditors of the company, hereby certify that so much of this report as related to the shares allotted, the cash received in respect of such shares, and the receipts and payments of the company are correct.
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


