Loading
Get National Cancer Institute Clinical Data Reporting Form - Lung 2008-2025
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the National Cancer Institute Clinical Data Reporting Form - Lung online
Filling out the National Cancer Institute Clinical Data Reporting Form - Lung is a crucial step in gathering important clinical data related to lung cancer. This guide provides clear, step-by-step instructions to help you complete the form accurately and efficiently, ensuring that you submit the required information online with confidence.
Follow the steps to successfully complete the form online:
- Press the ‘Get Form’ button to access the National Cancer Institute Clinical Data Reporting Form - Lung and open it in the designated online editor.
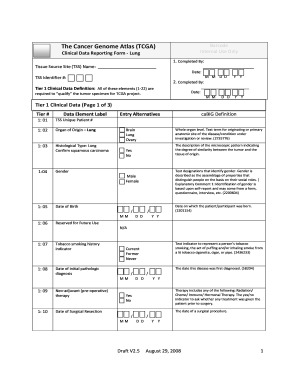
- Begin by filling in the Tissue Source Site (TSS) Name and TSS Identifier number at the top of the form.
- In the 'Completed By' section, provide your name and the date of completion.
- Continue with Tier 1 Clinical Data entries, starting with the TSS Unique Patient number, and ensure all required data elements are filled, including Organ of Origin and Histological Type.
- Fill in the dates related to the patient's birth, diagnosis, surgical procedures, and treatment history, ensuring all dates are in the correctly formatted MM/DD/YYYY.
- Provide detailed information about the patient’s tobacco smoking history and treatment options, selecting from the provided alternatives.
- Proceed with entering the Tumor stage and grade based on the AJCC system and include information regarding surgical margins.
- Finish filling out the remaining fields in Tier 1 and Tier 2 Clinical Data, providing detailed responses for additional treatments and patient's vital status.
- Once you have completed all sections, review the form for any missing or incorrect information.
- After verifying accuracy, save your changes, and choose to download, print, or share the form as necessary.
Ensure you complete the National Cancer Institute Clinical Data Reporting Form - Lung online today for effective clinical data management.
The targeted therapy Osimertinib (Tagrisso) was approved by the FDA in 2021 to be given after surgery—that is, as adjuvant therapy—to people with early-stage NSCLC that has certain mutations in the EGFR gene.
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


