Loading
Get Molarity Problems Worksheet
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the Molarity Problems Worksheet online
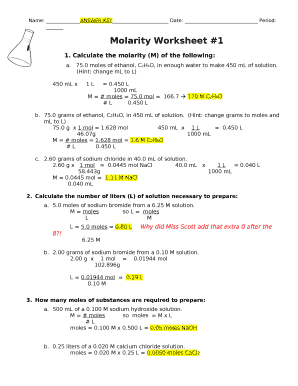
This guide provides step-by-step instructions for users filling out the Molarity Problems Worksheet online. Whether you're a student or an educator, this comprehensive overview will assist you in navigating the worksheet effectively.
Follow the steps to complete the Molarity Problems Worksheet seamlessly.
- Click ‘Get Form’ button to obtain the Molarity Problems Worksheet and open it in your preferred online editor.
- Begin by filling out the name and date fields at the top of the worksheet. Ensure that your name is provided clearly along with the date of completion.
- Move to the first problem. For each molarity calculation, identify the quantity of substance and convert any necessary units (e.g., from grams to moles, or milliliters to liters) as indicated by the hints in the problems.
- Apply the formula to calculate molarity (M). For instance, to find the molarity of ethanol, divide the number of moles by the volume in liters. Record your answer in the space provided.
- Continue through each problem sequentially. Pay attention to detail, ensuring accurate unit conversions and calculations as necessitated by each specific problem.
- If applicable, for problems asking for the preparation of a solution, calculate the required moles and grams based on the molarity and volume provided, and document your findings.
- After completing all problems, review your answers for accuracy and completeness.
- Finally, you can save changes, download, print, or share the completed Molarity Problems Worksheet as needed.
Start filling out your Molarity Problems Worksheet online for a clear understanding of molarity calculations!
Answer and Explanation: The concentration of the solution is 2.41 M.
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


