Loading
Get Crf Transmittal Form End Of Study Data - Planet 2
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the CRF TRANSMITTAL FORM End Of Study Data - Planet 2 online
Filling out the CRF Transmittal Form End Of Study Data - Planet 2 is a crucial task in ensuring that all necessary data is accurately recorded and submitted. This guide provides clear, step-by-step instructions to assist you in completing the form online.
Follow the steps to successfully complete the form.
- Use the ‘Get Form’ button to obtain the CRF Transmittal Form End Of Study Data - Planet 2. This will open the form in your online document editor.
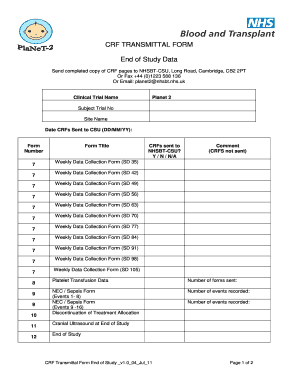
- Begin by entering the Clinical Trial Name, which is 'Planet 2'. This information is essential for identifying the study and ensuring the correct data association.
- Fill in the Subject Trial Number. Make sure this number corresponds accurately with the specific subject involved in the trial.
- Provide the Site Name where the trial is being conducted. This information helps in tracking the site of the data submission.
- Record the Date CRFs Sent to CSU (in the format DD/MM/YY). This date is important for compliance with submission timelines.
- For each form listed, indicate whether CRFs were sent to NHSBT-CSU by selecting 'Y', 'N', or 'N/A'. Use clear markings to avoid confusion.
- Include any comments in the designated area, especially if any CRFs were not sent, to clarify the situation.
- Count the number of forms sent and document this in the section provided for accurate reporting.
- For events recorded, input the number of NEC/Sepsis events for the patient clearly in the corresponding field.
- If applicable, fill out the totals for Platelet Transfusion Forms and NEC/Sepsis events for the patient.
- In the Confirmation of Receipt section, enter the Role and Name of the individual verifying the submission. This adds accountability to the process.
- Obtain and input the Signature and Date CRF receipt checked to authenticate the submission.
- After completing the form, save the changes, and consider downloading, printing, or sharing the form as necessary to maintain a record of your submission.
Complete your forms online today and ensure timely submission of your data.
At the end of the trial, CRF data from each study participant is fully analyzed to see whether the experimental medication worked. The compiled data results are documented in the clinical study report to provide the final results.
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


