Loading
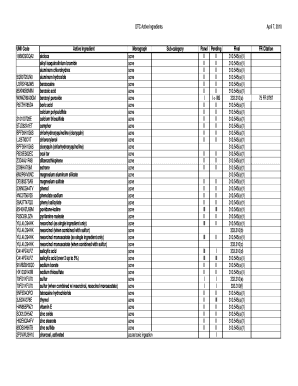
Get Otc Ingredient List Alphabetical By Monograph 4-07-10 - Fda
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the OTC Ingredient List Alphabetical By Monograph 4-07-10 - FDA online
This guide provides clear instructions on how to complete the OTC Ingredient List Alphabetical By Monograph 4-07-10 form online. By following these steps, users can efficiently fill out the form while ensuring compliance with the required standards.
Follow the steps to successfully complete the form.
- Click the ‘Get Form’ button to access the form and open it in your preferred format.
- Begin by filling in the UNII Code section. Each active ingredient has a unique identifier, which you will need to input accurately.
- Next, enter the Active Ingredient names as specified in the document. Ensure you use the correct spelling and terminology.
- In the Monograph section, select the corresponding monograph for each active ingredient used. This categorization is important for proper classification.
- Proceed to fill in the Sub-category for each ingredient, ensuring that it aligns with the intended use as specified in the guidelines.
- Once all necessary fields are filled out, review the information for accuracy and completeness. Double-check any references to coding or analytical standards.
- Finally, save your changes. You have the option to download, print, or share the completed form as needed.
Take action now and fill out your OTC Ingredient List online for efficient compliance management.
OTC Drug Approval and Monograph Requirements A primary difference is that approval of an NDA results in the approval to sell a specific finished drug product, whereas the OTC drug monograph process focuses on the safety and effectiveness of one or more active ingredients within a drug category.
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


