Loading
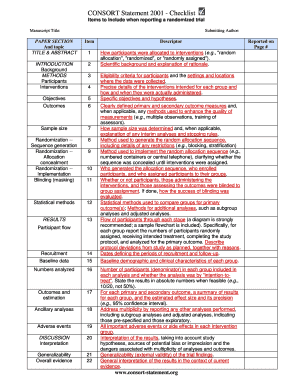
Get Consort Checklist Of Items To Include When Reporting A Randomized Trial Product Name
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to use or fill out the CONSORT Checklist of Items to Include When Reporting a Randomized Trial online
The CONSORT Checklist is a vital tool for researchers reporting randomized trials, ensuring that key information is clearly documented. This guide provides comprehensive, step-by-step instructions for completing the checklist online, supporting users in producing clear and consistent reports.
Follow the steps to effectively fill out the CONSORT Checklist online.
- Click ‘Get Form’ button to access the CONSORT Checklist and open it in the editor.
- Begin completing the form by entering the manuscript title and the submitting author details. Make sure to provide accurate and complete information.
- Fill in the abstract and title sections, summarizing the main findings of the trial in a concise manner, highlighting the objectives and outcomes.
- In the introduction section, outline the scientific background and the rationale for the trial, detailing the objectives and significance of the research.
- Complete the methods section, including participant eligibility criteria, interventions intended, and how they were administered.
- Detail the sampling methods, including how the sample size was determined, and describe the randomization process, allocation concealment, and implementation.
- Indicate whether blinding was used, and who was blinded during the trial to minimize bias in measurements and outcomes.
- Provide the statistical methods employed to analyze the data, ensuring clarity on groups compared for primary outcomes and any additional analyses performed.
- Move to the results section, documenting participant flow, recruitment data, and baseline characteristics of analyzed groups.
- Summarize the results for each primary and secondary outcome, including effect sizes and confidence intervals.
- Discuss the interpretation and generalizability of the findings in the context of existing literature.
- Upon completion of all sections, review the document for accuracy. You may save changes, download, print, or share the completed checklist.
Complete your CONSORT Checklist online today to enhance the quality of your trial reports.
The CONSORT diagram is a simple flow diagram showing the enrollment of subjects, their allocation to treatment, disposition status and how they are analysed in the trial. The layout of a CONSORT diagram depends on the study design.
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


