Loading
Get Spirit 2 Investigator Site File Checklist Generic Documents
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the SPIRIT 2 Investigator Site File Checklist Generic Documents online
This guide provides a clear and supportive approach to completing the SPIRIT 2 Investigator Site File Checklist for Generic Documents. Each section of the checklist will be analyzed, and users will receive step-by-step instructions to help ensure accurate completion.
Follow the steps to effectively fill out the checklist.
- Use the ‘Get Form’ button to obtain the SPIRIT 2 Investigator Site File Checklist and open it in an editor of your choice.
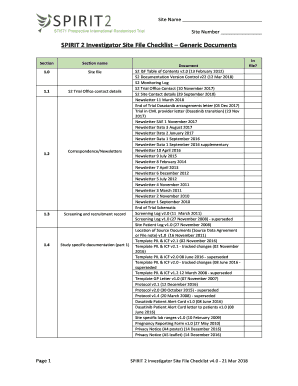
- Begin by entering the site name and site number in the designated fields at the top of the form. Ensure that all information is accurate, as it is crucial for identification purposes.
- Proceed to Section 1.1: S2 Trial Office contact details. Fill in all required contact information including phone numbers and email addresses, ensuring to verify its accuracy.
- Move to Section 1.2: Correspondence/Newsletters. Document all relevant newsletters and correspondence in the checklist, indicating the date and subject matter for each entry.
- In Section 1.3, provide details for the screening and recruitment record. This includes any records or logs of participants screened for the trial, with appropriate identification of each individual.
- For Section 1.4: Study specific documentation (part 1), list down all relevant documents specific to the trial that have been acquired. Ensure to organize them chronologically or by relevance.
- Continue through Section 1.5 and 1.6 for any additional study specific documentation and safety information, making sure to fill out as necessary.
- In Section 1.7, provide a list of research personnel involved in the trial, ensuring to include roles and responsibilities.
- In the final sections, document any central approvals received, along with relevant correspondence and safety information according to the structured format provided.
- Once all sections are completed, review the entire form for any discrepancies or missing information. Save your changes and download a copy of the completed form for your records.
Complete your SPIRIT 2 Investigator Site File Checklist online today to ensure efficient document management.
Related links form
In clinical research, essential documents are defined as documents that “individually and collectively permit evaluation of the conduct of a trial and the quality of the data produced.” Not all essential documents will be available at the beginning of a study, and not all documents have to be stored in the same ...
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


