Loading
Get Medical Device Adverse Event Reporting Form - Indian ... - Ipc Nic
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the Medical Device Adverse Event Reporting Form - Indian Ipc Nic online
This guide provides a comprehensive overview of how to complete the Medical Device Adverse Event Reporting Form. By following these steps, you can efficiently submit a report regarding adverse events related to medical devices.
Follow the steps to fill out the Medical Device Adverse Event Reporting Form online.
- Press the ‘Get Form’ button to access the Medical Device Adverse Event Reporting Form and open it in your selected editor.
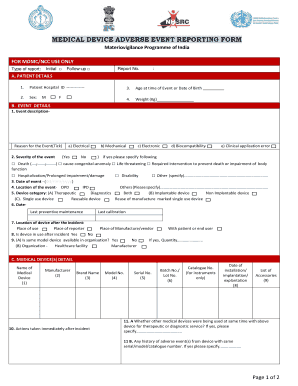
- Begin by completing the patient details section. Input the patient hospital ID, age at the time of the event or date of birth, sex, and weight.
- In the event details section, provide a thorough description of the event. Indicate the reason for the event by ticking the appropriate box and assess the severity. Be sure to include the date and location of the event.
- Identify the device category by selecting from the options provided, such as therapeutic, diagnostic, implantable, or non-implantable. Also, specify whether the device is single-use or reusable.
- Fill in the medical device details section, including the name, manufacturer, brand name, model number, serial number, and any relevant accessory information.
- Document any actions taken immediately following the incident. If other medical devices were used concurrently, specify them as needed.
- Complete the regulatory details by providing the manufacturer’s name and other required regulator information.
- In the reporter details of the MvPI centre section, enter your professional address, contact information, and designation. Ensure the signature and date of the report are included.
- Finally, review all sections for accuracy. Once satisfied, you can save changes, download, print, or share the completed form as necessary.
Complete the Medical Device Adverse Event Reporting Form online today to ensure quick and efficient reporting.
Related links form
Mandated Timeliness For Medical Device Reporting Importers must submit a report within 30 days of becoming aware of a reportable event. Once a medical device manufacturer becomes aware of a reportable death, serious injury or malfunction, it has 30 days to report the adverse event to the FDA.
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


